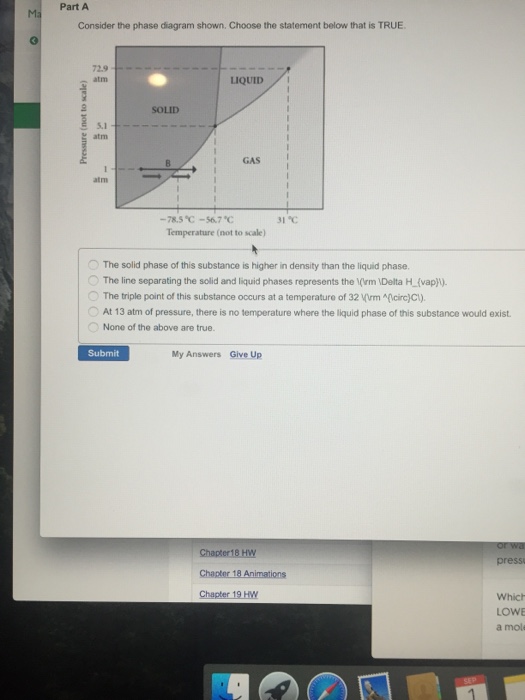
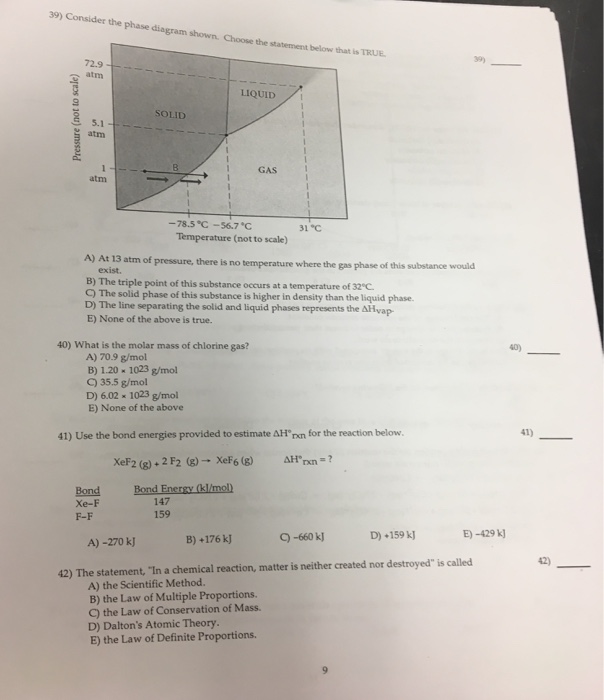
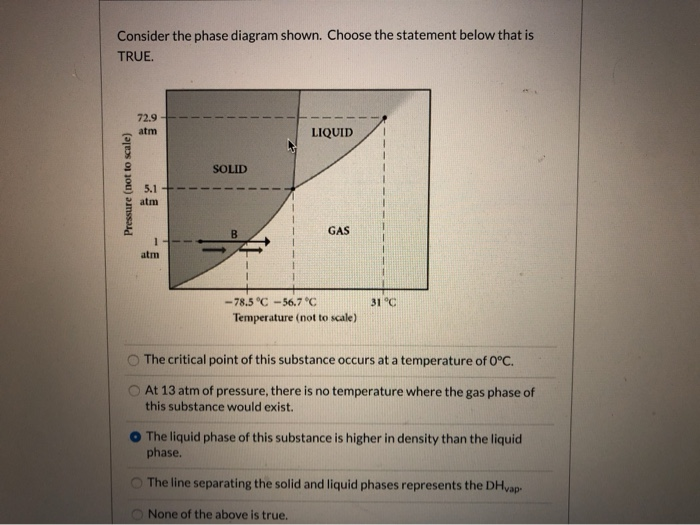
37 consider the phase diagram shown. choose the statement below that is true.
4. Consider the phase diagram below. If the dashed line at 1 atm of pressure is followed from 100 to 500°C, what phase changes will occur (in order of increasing temperature)? A) condensation, followed by vaporization B) sublimation, followed by deposition C) vaporization, followed by deposition D) fusion (melting), followed by vaporization 88) Assign the appropriate labels to the phase diagram shown below. B) A = gas, B = solid, C = liquid, D = triple point: 89) Consider the phase diagram shown. Choose the statement below that is TRUE. C) The solid phase of this substance is higher in density than the liquid phase. 90) Consider the phase diagram below.
21. Consider the phase diagram shown. Choose the statement below that is TRUE. A) The triple point of this substance occurs at a temperature of 31°C. B) At 10 atm of pressure, there is no temperature where the liquid phase of this substance would exist. C) The solid phase of this substance is higher in density than the liquid phase.

Consider the phase diagram shown. choose the statement below that is true.
Thus, the balanced voltages yield balanced currents. The phasor diagram for the line currents and the line-to-neu tral voltages is shown below. If we lay the line-to-neutral voltage phasors end to end, they form a closed triangle (the same property is true for the line currents). The closed triangle shows that the sum of these phasors is zero. A phase diagram is a graphical representation of pressure and temperature of a material. Phase diagrams show the state of matter at a given pressure and temperature. They show the boundaries between phases and the processes that occur when the pressure and/or temperature is changed to cross these boundaries. Thank you! Select all statements that are TRUE, when considering the phase diagram shown. 10- atm B A 31 C -56 C Temperature (not to scale) Pressure ...1 answer · 0 votes: The last option is true. P Soid gas slople SH 2 Now; solid Ri equiibrumj From the Densin id Greater han SOlid Proeers , uwd Condensation Pis a roiple ...
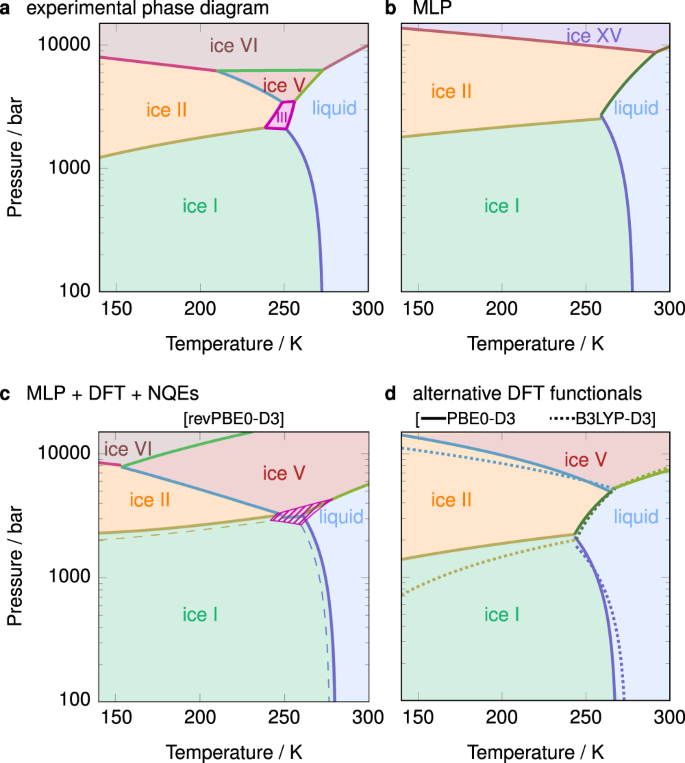
Consider the phase diagram shown. choose the statement below that is true.. Consider the phase diagram for carbon dioxide shown in Figure 5 as another example. The solid-liquid curve exhibits a positive slope, indicating that the melting point for CO 2 increases with pressure as it does for most substances (water being a notable exception as described previously). Notice that the triple point is well above 1 atm ... Transcribed image text: Consider the phase diagram shown. Choose the statement below that is TRUE. The solid phase of the substance is higher in density than the liquid phase. The line separating the solid and liquid phases represents the \ (\rm Delta H_ (vap)\). The triple point of this substance occurs at a temperature of 32 \ (\rm^ (\circ)C\). (numbers on phase diagram correspond with numbers below) 1. Assume a melt comp- osition of 70% B and 30% A. 2. Cool melt to liquidus line along red arrow. Only B crystals form at about 1380 o (B is immiscible with A). Only B crystallizes because we are on the Melt+B liquidus line; no A can crystallize until the eutectic is reached. Phase Diagram: In this phase diagram, which is typical of most substances, the solid lines represent the phase boundaries.The green line marks the freezing point (or transition from liquid to solid), the blue line marks the boiling point (or transition from liquid to gas), and the red line shows the conditions under which a solid can be converted directly to a gas (and vice-versa).
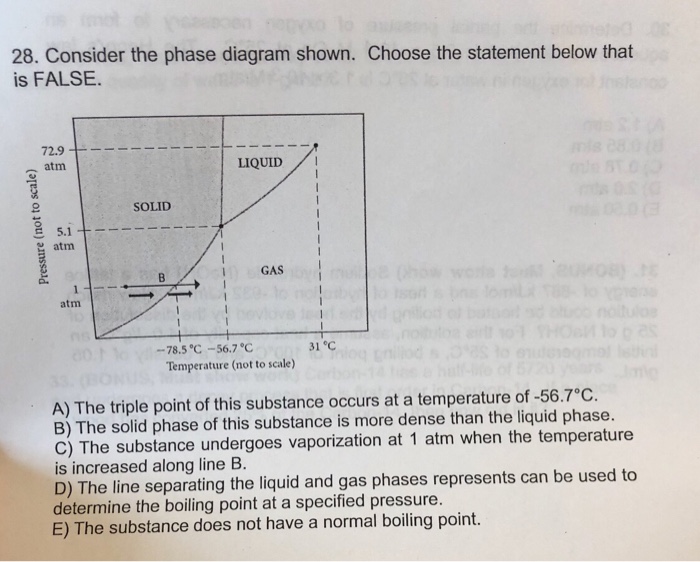
9) 9) Consider the phase diagram shown. Choose the statement below that is TRUE 72.9 atm LIQUID SOLID atim GAS atm -78.5â -56.7â 31â Temperature (not to scale) A) The solid phase of this substance is higher in density than the liquid phase. Phase diagrams. A phase diagram lets you work out exactly what phases are present at any given temperature and pressure. In the cases we'll be looking at on this page, the phases will simply be the solid, liquid or vapour (gas) states of a pure substance. This is the phase diagram for a typical pure substance. Choose the statement below that is TRUE 2 atm LIQUID SOLID o am GASI atm -78.5 ?-56.7 °C 31°C Temperature (not to scale) a. The line separating the solid and ... Below is shown the titanium-copper phase diagram (Figure 9.37). There is one eutectic on this phase diagram, which exists at about 51 wt% Cu-49 wt% Ti and 960°C. Its reaction upon cooling is 2 L! TiCu + TiCu There is one eutectoid for this system. It exists at about 7.5 wt% Cu-92.5 wt% Ti and 790°C. This reaction upon cooling is ! " # + Ti2Cu
Phase Diagrams • Indicate phases as function of T, Co, and P. • For this course:-binary systems: just 2 components.-independent variables: T and Co (P = 1 atm is almost always used). • Phase Diagram for Cu-Ni system Adapted from Fig. 9.3(a), Callister 7e. (Fig. 9.3(a) is adapted from Phase Diagrams of Binary Nickel Alloys , P. Nash of all elements that belong to both A and B. This is what the two sets have in common. Below is a venn diagram illustrating the set A\B. A B A\B Complement of a Set If U is a universal set and A is a subset of U,thenthesetofallelements in U that are not in A is called the complement of A and is denoted Ac.Belowarevenndiagrams Meaning of Phase Diagram: A phase diagram is also called an equilibrium or constitutional diagram. It shows the relationship between temperature, the compositions and the quantities of phases present in an alloy system under equilibrium conditions. When temperature is altered many microstructure develop due to phase transformation. What can be proven true in the diagram is the statement in letter C. angle CGB is complementary to angle AGD. It is because they both had the same vertex which is point G. Complementary angles are angles which are equal to 90 degrees wherein when you add both angles which are obviously 45 degrees, the sum is 90 degrees.
Consider the phase diagram for carbon dioxide shown in Figure 5 as another example. The solid-liquid curve exhibits a positive slope, indicating that the melting point for CO 2 increases with pressure as it does for most substances (water being a notable exception as described previously). Notice that the triple point is well above 1 atm, indicating that carbon dioxide cannot exist as a liquid ...
Indicate whether each of the following statements is true or false: (1) _____ The system is never an actor in a Use Case Diagram. (2) ... Consider the following UML Activity Diagram. ... Use Case Descriptions are more appropriate than Activity Diagrams for this purpose during the product design phase. (Hint: Think about how they are used and ...
Consider the phase diagram shown. Choose the statement below that is TRUE. A) The triple point of this substance occurs at a temperature of 31°C. B) At 10 atm of pressure, there is no temperature where the liquid phase of this substance would exist. C) The solid phase of this substance is higher in density than the liquid phase.
Consider the phase diagram shown. Choose the statement below that is TRUE. A) The triple point of this substance occurs at a temperature of 31°C. B) At 10 atm of pressure, there is no temperature where the liquid phase of this substance would exist. C) The solid phase of this substance is higher in density than the liquid phase.

Thermodynamic Analyses Of Fuel Production Via Solar Driven Non Stoichiometric Metal Oxide Redox Cycling Part 1 Revisiting Flow And Equilibrium Assumptions Energy Fuels
ANS: D PTS: 1 DIF: moderate REF: 11.3 OBJ: Define phase diagram. TOP: phases | phase transitions 31. Choose the correct statement about the diagram below. A) The diagram shows the triple point above 1 atm pressure. B) The diagram is qualitatively correct for water. C) The diagram shows that the melting point of the solid increases with ...
Shown below is the Cu-Ni phase diagram (Figure 9.3a) and a vertical line constructed at a composition of 70 wt% Ni-30 wt% Cu. (a) Upon heating from 1300°C, the first liquid phase forms at the temperature at which this vertical line intersects the α-(α + L) phase boundary--i.e., about 1345°C. )-)-
Consider the phase diagram for carbon dioxide shown in as another example. The solid-liquid curve exhibits a positive slope, indicating that the melting point for CO 2 increases with pressure as it does for most substances (water being a notable exception as described previously). Notice that the triple point is well above 1 atm, indicating ...
Choose the statement below that is TRUE. A) A solution will form between two substances if the solute-solvent interactions are of comparable strength to the solute-solute and solvent-solvent interactions. ... Assign the appropriate labels to the phase diagram shown below. A) A = liquid, B = solid, C = gas, D = critical point ... Consider the ...

Kdoqi Clinical Practice Guideline For Vascular Access 2019 Update American Journal Of Kidney Diseases
18 Nov 2019 — Get the detailed answer: 9) 9) Consider the phase diagram shown. Choose the statement below that is TRUE 72.9 atm LIQUID SOLID atim GAS atm ...
Phase Diagrams QUIZ DRAFT. 10th - 12th grade. 380 times. Chemistry. 59% average ... Q. True or false: melting and freezing occur at the same temperature! answer choices . True. False. I don't know. Tags: Question 4 . SURVEY . 45 seconds . Q. A substance's heating curve is shown in the graph. What is its boiling point? (Diagram B) answer choices ...
Consider the phase diagram shown: Choose the statement below that is TRUE QUID Soic GaS 785 C 56.7 € JIc Temperature (fol to scale} The Pc on this ...4 answers · Top answer: a here is false. Ah, because that occurs at approximately 265 Kelvin. Part B is also false ...
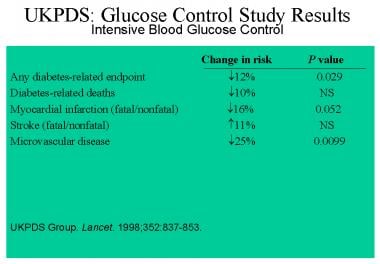
Type 2 Diabetes Mellitus Treatment Management Approach Considerations Pharmacologic Therapy Management Of Glycemia
The phase diagram shows, in pressure–temperature space, the lines of equilibrium or phase boundaries between the three phases of solid, liquid, and gas.
Consider the phase diagram shown below. Choose the statement below that is TRUE. (A) The triple point of this substance occurs at a temperature of 72.9 atm. (B) At 10 atm of pressure, there is no temperature where the liquid phase of this substance would exist.
Use the phase diagram above to answer the questions below .( Remember that standard pressure is 1.00 atm and it's used to find normal phase change temperatures.) 1: In which state/phase of matter is this substance at -10 °C and a pressure of 0.50 atm? 2: What phase change occurs as the substance at 0.75 atm is heated from 0°C to 25°C
1. Refer to the phase diagram shown. The region enclosed by points DEF can be described as a (A) mixture of solid [3 component and liquid a component (B) mixture of solid f3 and liquid (3 component (C) peritectic composition (D) mixture of solid (3 component and a molten mix ture of a and (3 components
Consider the phase diagram shown. Choose the statement below that is TRUE. A) The triple point of this substance occurs at a temperature of 31°C. B) At 10 atm of pressure, there is no temperature where the liquid phase of this substance would exist. C) The solid phase of this substance is higher in density than the liquid phase.
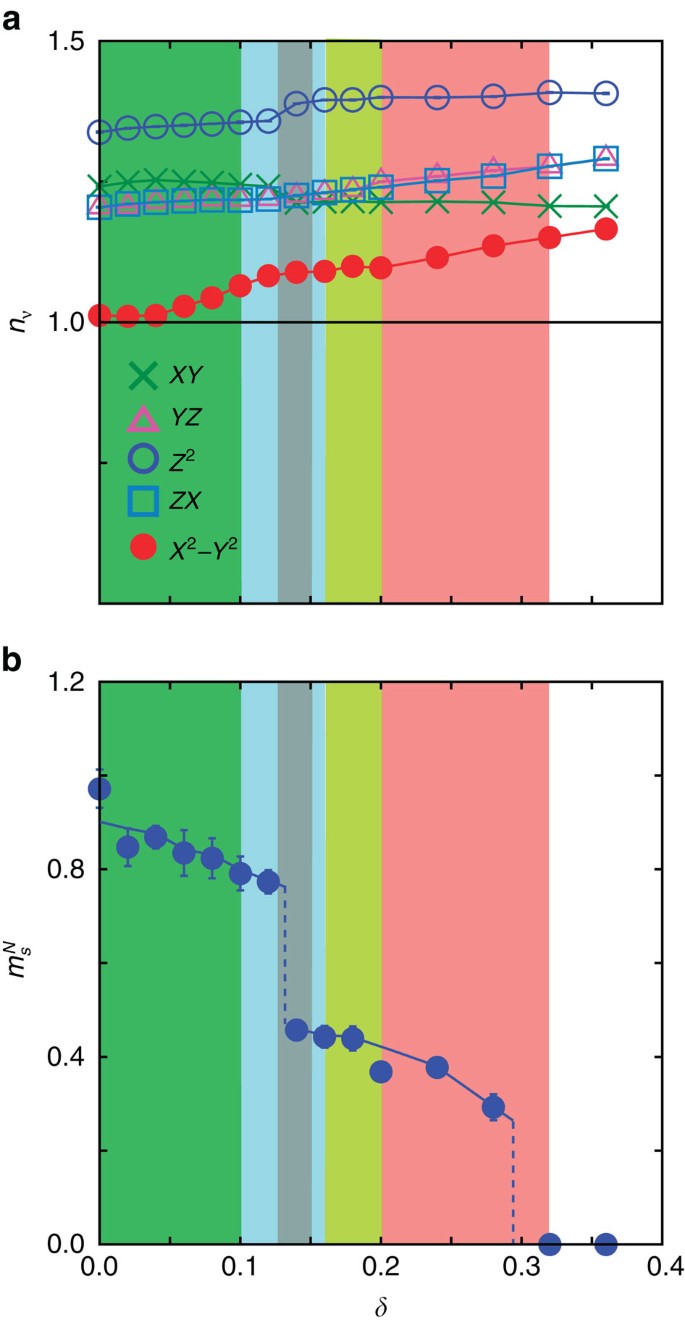
Superconductivity And Its Mechanism In An Ab Initio Model For Electron Doped Lafeaso Nature Communications
22) Consider the phase diagram shown. Choose the statement below that is true.A) The triple point of this substance occurs at a temperature of 31°C.B) At 10 ...
9) 9) Consider the phase diagram shown. Choose the statement below that is TRUE 72.9 atm LIQUID SOLID atim GAS atm -78.5â -56.7â 31â Temperature (not to scale) A) The solid phase of this substance is higher in density than the liquid phase.
Thank you! Select all statements that are TRUE, when considering the phase diagram shown. 10- atm B A 31 C -56 C Temperature (not to scale) Pressure ...1 answer · 0 votes: The last option is true. P Soid gas slople SH 2 Now; solid Ri equiibrumj From the Densin id Greater han SOlid Proeers , uwd Condensation Pis a roiple ...
A phase diagram is a graphical representation of pressure and temperature of a material. Phase diagrams show the state of matter at a given pressure and temperature. They show the boundaries between phases and the processes that occur when the pressure and/or temperature is changed to cross these boundaries.
Thus, the balanced voltages yield balanced currents. The phasor diagram for the line currents and the line-to-neu tral voltages is shown below. If we lay the line-to-neutral voltage phasors end to end, they form a closed triangle (the same property is true for the line currents). The closed triangle shows that the sum of these phasors is zero.

The Diagram Goes With The Questions Below For Problem 7 9 On The Phase Diagram Shown Above Segment Corresponds Homeworklib

Question 22 Consider The Following Phase Diagram For A Hypothetical Substance Which Arrow Corresponds To The Homeworklib
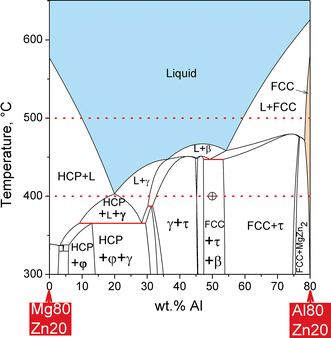











0 Response to "37 consider the phase diagram shown. choose the statement below that is true."
Post a Comment