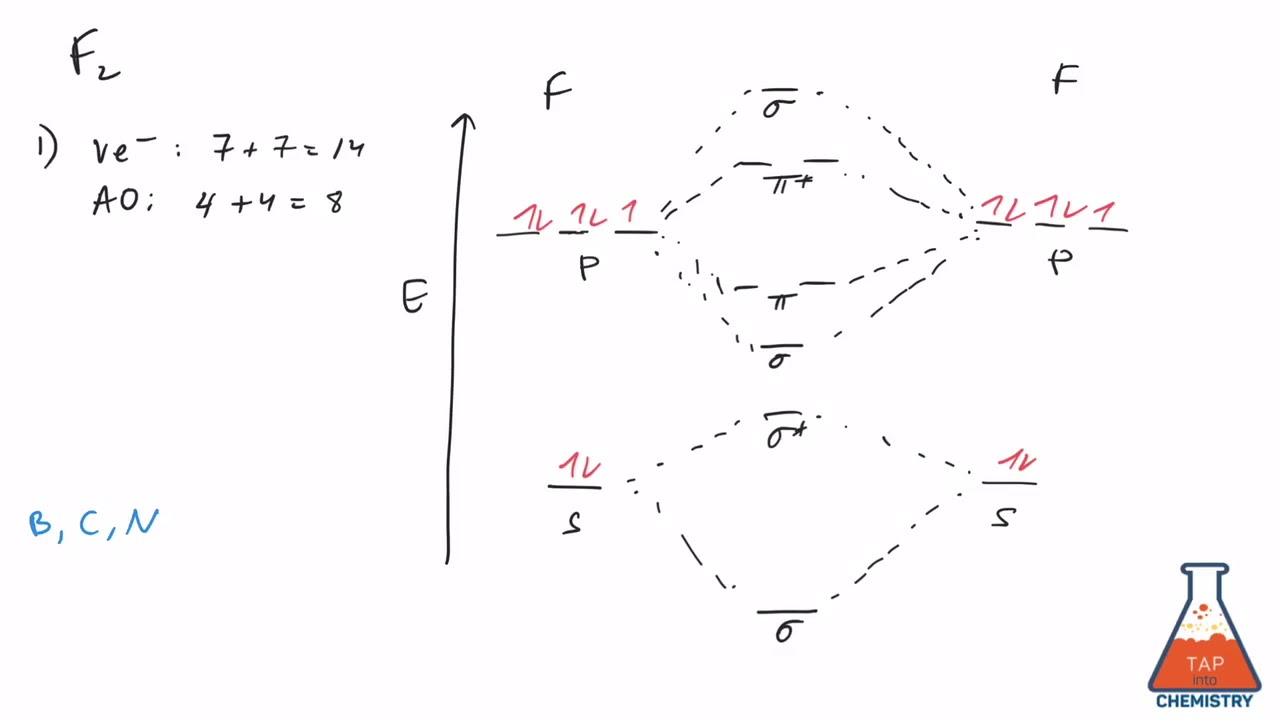
37 f2+ molecular orbital diagram
Nov 02, 2021 · XeF2 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram XeF2 is a covalent inorganic halide formed by the inert gas xenon and the halogen fluorine. This is an active solvent and is found to be soluble in different fluorides like HF and bromine pentafluoride. 15 F2 Molecular Orbital Diagram. We assume that the electrons would fill the molecular orbitals of molecules like electrons fill atomic we will use this diagram to describe o2, f2, ne2, co, and no. The lowest energy unoccupied molecular orbital is 2p_ (sigma), so that is where the extra electron will be added.
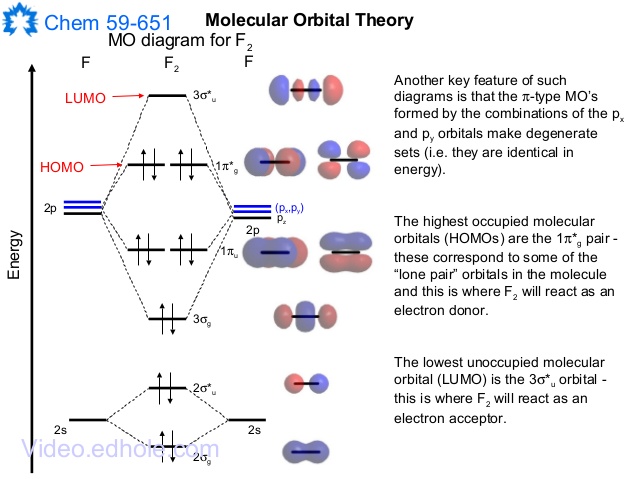
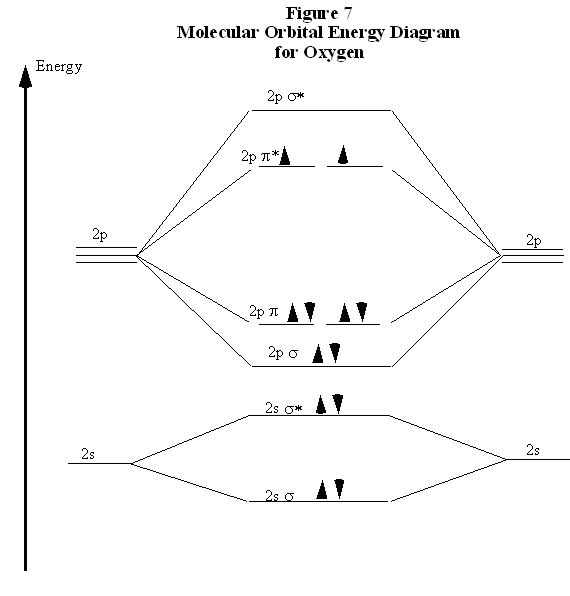
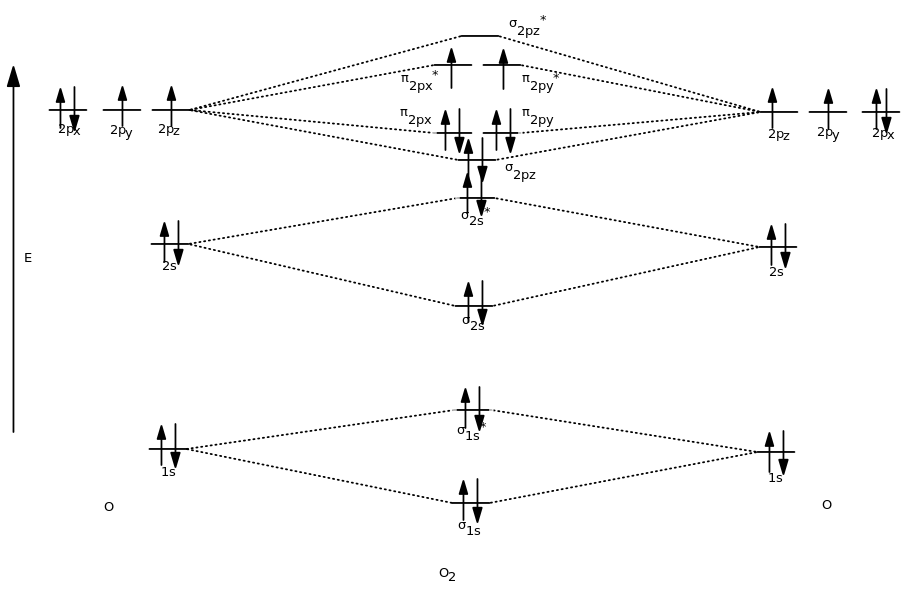
Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ...

F2+ molecular orbital diagram
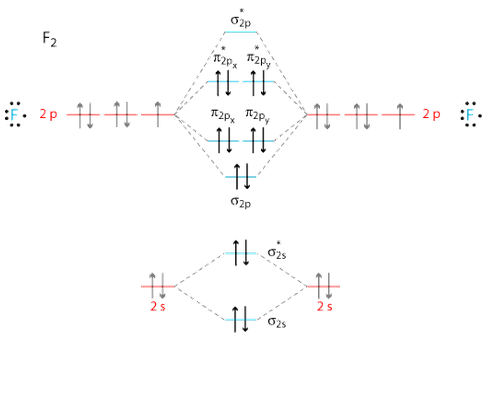
#sigma# molecular orbitals are singly-degenerate, and #pi# molecular orbitals are doubly-degenerate. #sigma# molecular orbitals, in principle, get more stabilized upon overlap than #pi# molecular orbitals do. For example, an #ns//ns# overlap for a homonuclear diatomic molecule gives rise to a partial MO diagram like this: Molecular orbital diagram for f2. In o 2 and f 2 there is a crossover of the sigma and the pi ortbials. Molecular orbitals mo are constructed from atomic orbitals. The relative energies of the sigma orbitals drop below that of the pi orbitals. The size of the effect depends on the 2s 2p energy difference. It is called a sigma molecular orbital ... Molecular orbital diagram for f2. A draw the molecular orbital diagram. They also give insight to the bond order of the molecule how many bonds are shared between the two atoms. The relative energies of the sigma orbitals drop below that of the pi orbitals. The molecular orbital theory mo has been introduced for the diatomic hydrogen molecules.
F2+ molecular orbital diagram. Molecular Orbital Diagram - Cl2, Br2, I2 3s & 3p and higher atomic orbitals are not so widely separated in energy and allow significant mixing (hybridization) to occur. This mixing causes the inversion of the σσand πmolecular orbitals' energy. σσσ ππ σ* π* 3,4,5 p 3, 4,5 s σ* σ 3,4,5 s 3,4,5 p Interhalogens Br Br F F Br F F F F. Molecular orbital diagram for f2. A fundamental principle of these theories is that as atoms bond to form molecules a certain number of atomic orbitals combine to form the same number of. C would this ion exist. To further demonstrate the consistency of the lewis structures with mo. For the ion f2. May 01, 2021 · Molecular Orbital Electronic Configurations: Molecular orbital electronic configurations of some molecules/ions are given below: → Hydrogen Bond: The attractive force which binds the hydrogen atom of one molecule with the electronegative atom (F, O, N) of another molecule is known as a hydrogen bond. A hydrogen bond is weaker than a covalent ... Answer (1 of 6): Here is the solution, > * For O2 molecule, > * For F2 molecule, Thanks for reading.
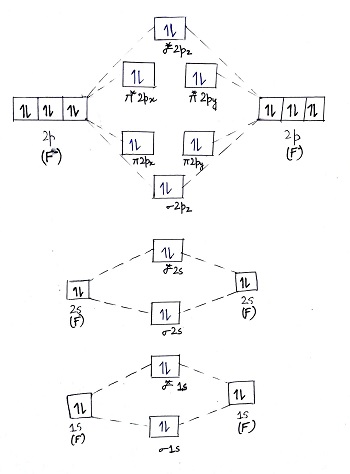
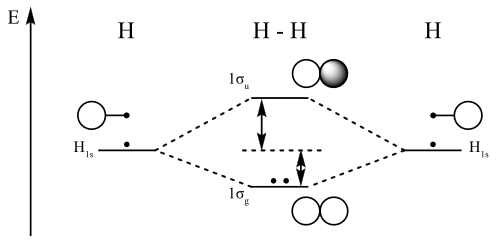
Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules An atomic orbital is located on a single atom. When two (or more) atomic orbitals overlap to make a bond we can change our perspective to include all of the bonded atoms and their overlapping orbitals. Since more than one atom is involved, we refer to these orbitals as molecular orbitals. Answer (1 of 4): The atomic number of fluorine is 9, so a (neutral) F2 molecule has a total of 18 electron, or 14 valence electrons (excluding the four 1s electrons). The (F2)- ion has one more valence electron, or 15. The orbital diagram for a diatomic molecule is To find the bond order, add th... Solved Complete the molecular orbital diagram of F2 and F2. | Chegg.com. Science. Chemistry. Chemistry questions and answers. Complete the molecular orbital diagram of F2 and F2. What is the bond order of F2? Oo 0.5 1.5 O O Complete the molecular orbital diagram of F2 and F2. What is the bond order of F2? 1.5 O 0.5. Molecular Orbital Diagram For F2 - A Mechanistic Study Graphene Based Nonvolatile Reram Devices. molecular orbitals of diatomic molecules molecular orbitals of li 2 be 2 to f 2 skills to develop explain how the energy levels of atomic orbitals vary for h li be b c n and o draw relative energy levels diagrams for homonuclear diatomic molecules ...
Solved question 1 by drawing molecular orbital diagrams solved look at the mo diagrams of corresponding neutral diatom when doing molecular orbitals the pi bonds come before sigma for b2 what is the energy level diagram of n2 and f2 brainly in. The case of F2 is a simple one because of the symmetry and diatomicity of the molecule. In more complex molecules (polyatomic and asymmetric), the extent of mixing and thus the contribution of individual atomic orbitals to form a particular molecular orbital depends on the relative energy alignment of the atomic orbitals. F2 Polarity Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in Nov 21, 2018 · Figure A partial molecular orbital energy-level diagram for the HF molecule. This interaction introduces an element of s-p mixing, or hybridization, into the molecular orbital theory. The result is a slight change in the relative energies of the molecular orbitals, to give the diagram shown in the figure below.
This page is based on the copyrighted Wikipedia article "Molecular_orbital_diagram" ; it is used under the Creative Commons Attribution-ShareAlike 3.0 Unported License. You may redistribute it, verbatim or modified, providing that you comply with the terms of the CC-BY-SA.
Chemistry questions and answers. Complete the molecular orbital diagram of F2 and F2-. What type of orbital contains the highest energy electron (s) in F2? pi, antibonding sigma, bonding sigma, antibonding pi, bonding Which atom is larger in size (radius), Cr or Cr3+? Question: Complete the molecular orbital diagram of F2 and F2-.
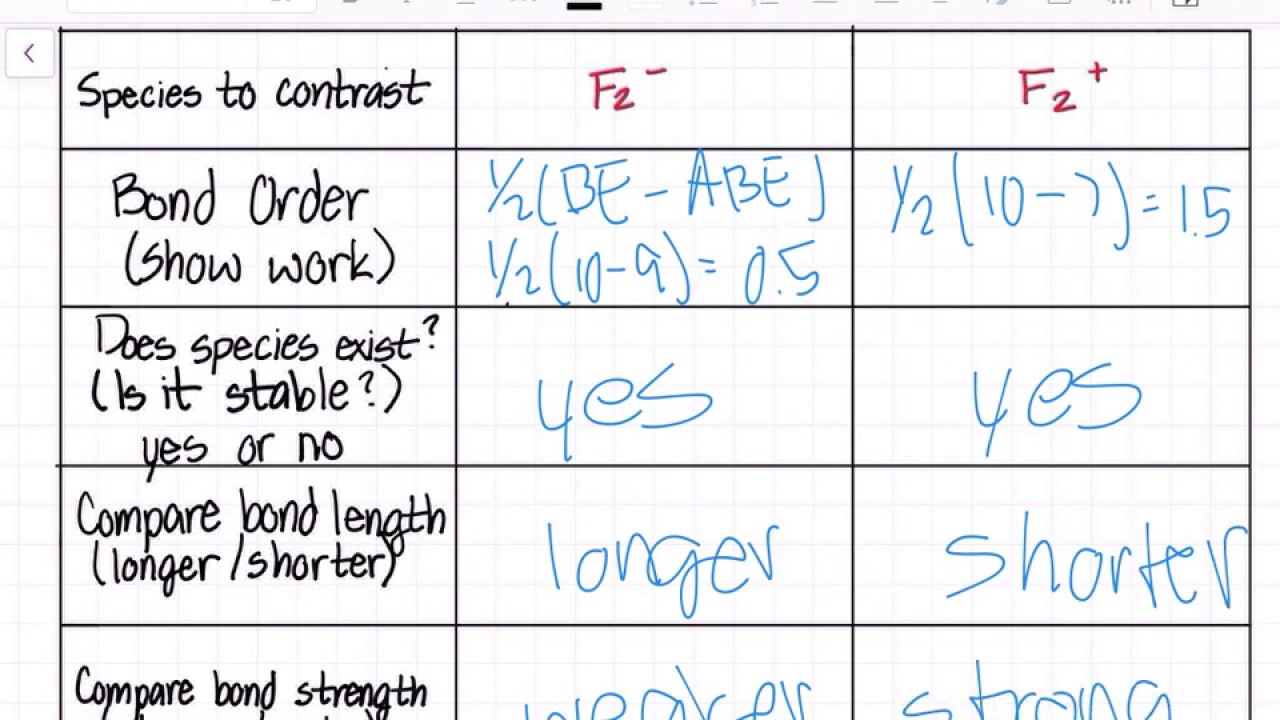
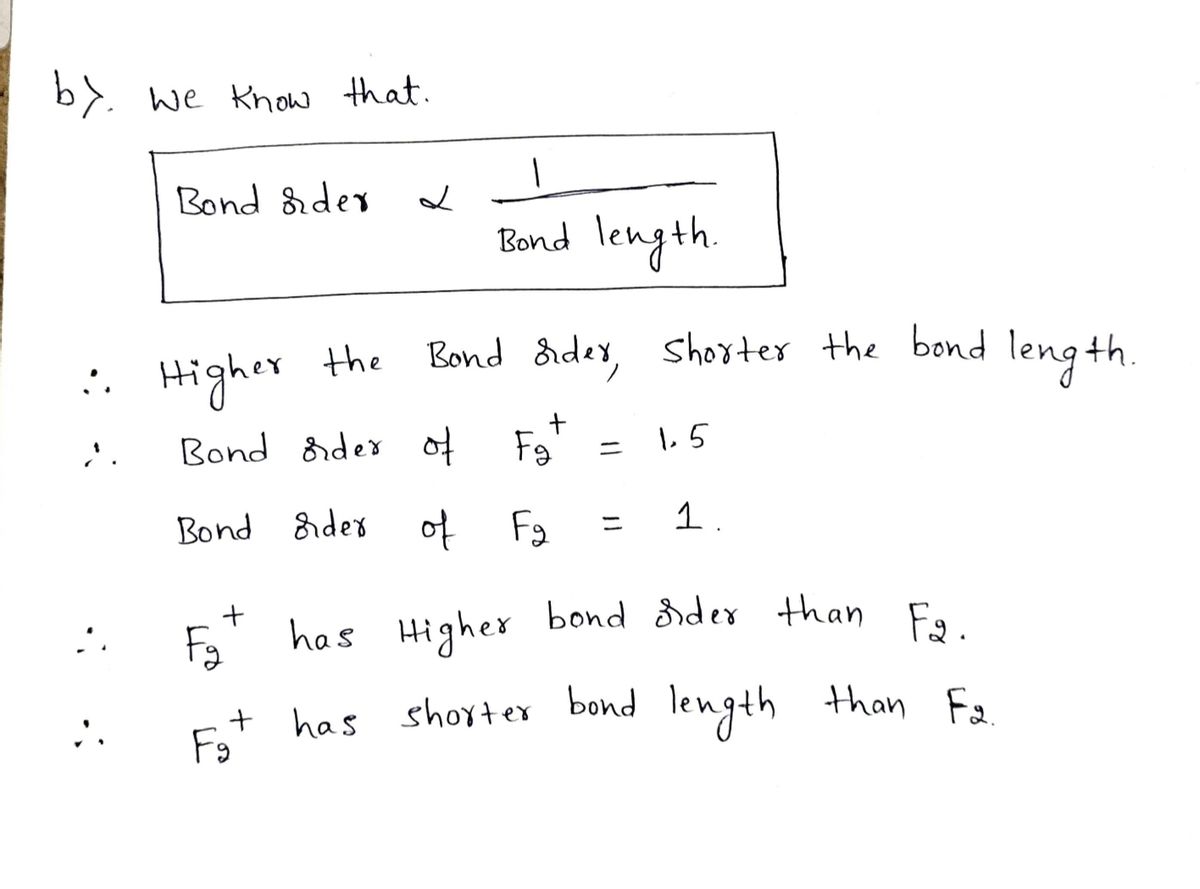
Arrange the following in order of decreasing stability. a blank molecular orbital diagram (part a 1 figure) has been provided to you. rank the fluorine species from most to least stable. to rank items as equivalent, overlap them. f2, f2+, f2-
Printable O2 molecular orbital diagrams are available for you to guide your study in the molecular orbital lesson.This diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of a molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.
When two fluorine atoms bond, the sigma(2p) bonding molecular orbitals are lower in energy than the pi(2p) bonding orbitals.F2(2+) has a bond order of 2, so ...
The molecular orbital diagram for an o 2 molecule would therefore ignore the 1s electrons on both oxygen atoms and concentrate on the interactions between the 2s and 2p valence orbitals. F2 molecular orbital diagram. Atomic oxygen stock alamy. Organic chemistry lone pairs bonding pi molecular orbitals. F2 molecular orbital theory.
Molecular orbital diagram for f2. A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. How to graph a mo molecular orbital diagram for f2. By drawing molecular orbital diagrams for b2 c2 n2 o2 and f2 predict which of these homonuclear diatomic molecules are magnetic.
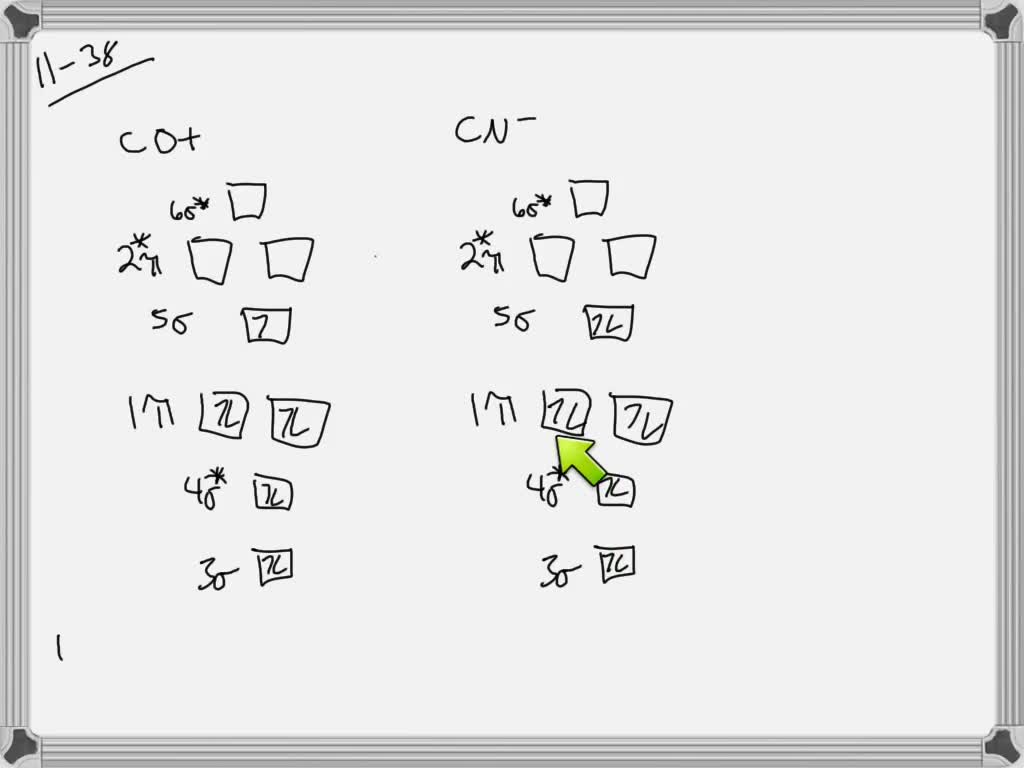
For the ion F2+:a) Draw the molecular orbital diagram.b) Calculate the bond order.c) Would this ion exist?d) Write the electron configuration of the ion.————...
Solved question 1 by drawing molecular orbital diagrams solved look at the mo diagrams of corresponding neutral diatom when doing molecular orbitals the pi bonds come before sigma for b2 what is the energy level diagram of n2 and f2 brainly in. Information from the mo diagram justify o2s stability and show that its bonding order is 2.
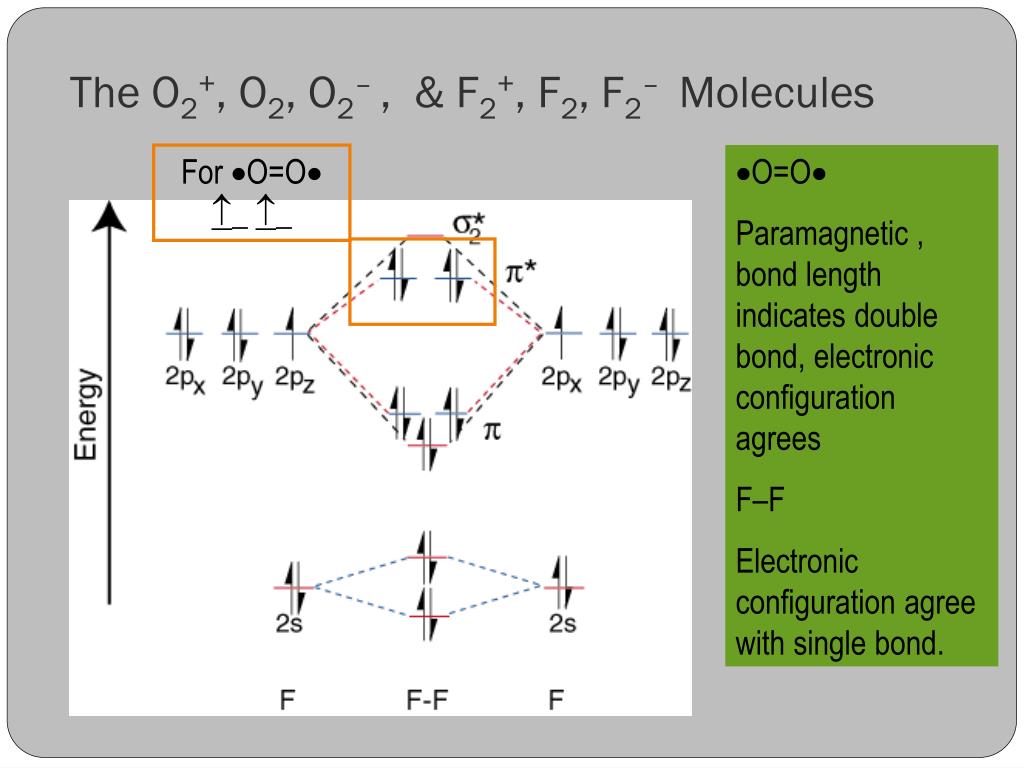
Relationship between electronic configuration and Molecular behaviour. 1) Stability of molecules in terms of bonding and antibonding electrons . Number of electrons present in the bonding orbitals is represented by N b and the number of electrons present in antibonding orbitals by Na.. 1) If N b > Na,the molecule is stable because greater number of bonding orbitals are occupied than ...
26. Define anti-bonding molecular orbital. Ans: This can be defined as the molecular orbital which is formed by the subtraction of atomic orbitals is called anti-bonding molecular orbital. This is represented as: ${{\sigma }^{+}}=\Psi A-\Psi B$ 27. Explain diagrammatically the formation of molecular orbital by LCAO.
Solved Use Molecular Orbital Theory To Predict The Following Properties Of The F2 Ion A Electron Configuration B Bond Order C Magnetic Character Paramagnetic Or Diamagnetic D Whether The Bond Length Is Longer
Click here👆to get an answer to your question ️ 37. Draw molecular orbital diagram for F2 molecule. Also, give its electronic configuration, bond order and magnetic property. 138. Solve the following:
Problem: Use the molecular orbital diagram shown to determine which of the following is most stable.a. F22+b. Ne22+c. F22-d. O22+e. F2
Atoms of which element, indicated by letter on the periodic table, have the orbital-filling diagram shown below? A What is the de Broglie wavelength of an electron (m = 9.11 × 10-31 kg) moving at a velocity of 3.0 × 107 m/s (10% of the speed of light)?
Molecular Orbital Diagram for the HF Molecule Interaction occurs between the 1s orbital on hydrogen and the 2p orbital in fluorine causing the formation of a sigma-bonding and a sigma-antibonding molecular orbital, as shown below. Figure 1: Molecular orbitals of HF. (CC BY-SA-NC 2.0 UK: England & Wales License; Nick Greeves).

Write Molecular Orbital Configuration Of C2 Predict Magnetic Behaviour And Calculate Its Bond Order H7ch14qq Chemistry Topperlearning Com
A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. Molecular orbital diagram for f2. The other molecular orbital produced s h h shows a decrease in electron density between the nuclei reaching a value of zero at the midpoint between the nuclei where there is a nodal plane.
Write molecular orbital configuration of c2 predict magnetic behaviour and calculate its bond order energy levels of molecular orbitals for the second row diatomic molecules a n2 b o2 and c f2 mo diagram for formation of nitrogen molecule from atoms atomic and molecular (Select] 0.
Draw molecular orbital diagram for F 2 molecule. Also, gives its electronic configuration, bond order and magnetic property. Hint: The Molecular Orbital Theory (MOT) explains the formation of the molecule in a better way than Valence Bond Theory (VBT). The bond order calculations are feasible using MOT and so is the description of electronic ...
Molecular orbital diagram for f2. A draw the molecular orbital diagram. They also give insight to the bond order of the molecule how many bonds are shared between the two atoms. The relative energies of the sigma orbitals drop below that of the pi orbitals. The molecular orbital theory mo has been introduced for the diatomic hydrogen molecules.
Molecular orbital diagram for f2. In o 2 and f 2 there is a crossover of the sigma and the pi ortbials. Molecular orbitals mo are constructed from atomic orbitals. The relative energies of the sigma orbitals drop below that of the pi orbitals. The size of the effect depends on the 2s 2p energy difference. It is called a sigma molecular orbital ...
#sigma# molecular orbitals are singly-degenerate, and #pi# molecular orbitals are doubly-degenerate. #sigma# molecular orbitals, in principle, get more stabilized upon overlap than #pi# molecular orbitals do. For example, an #ns//ns# overlap for a homonuclear diatomic molecule gives rise to a partial MO diagram like this:
















0 Response to "37 f2+ molecular orbital diagram"
Post a Comment