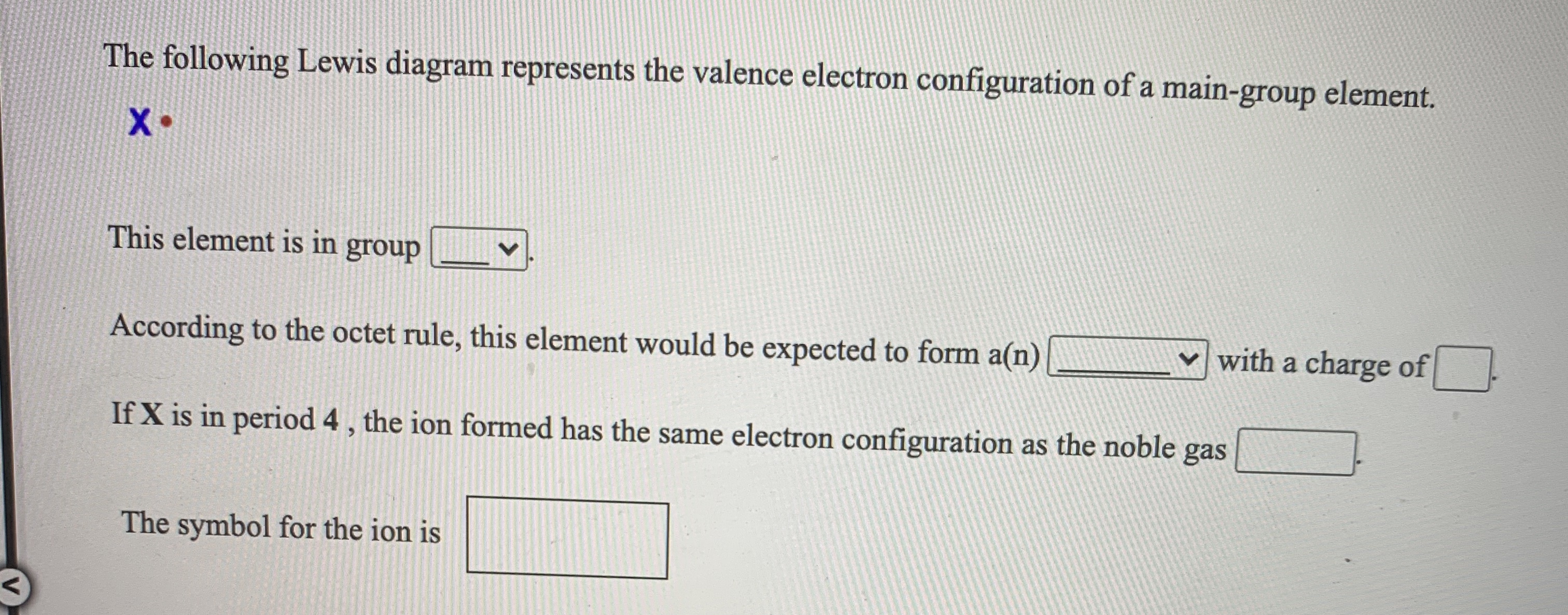
37 the following lewis diagram represents the valence electron configuration of a main-group element.
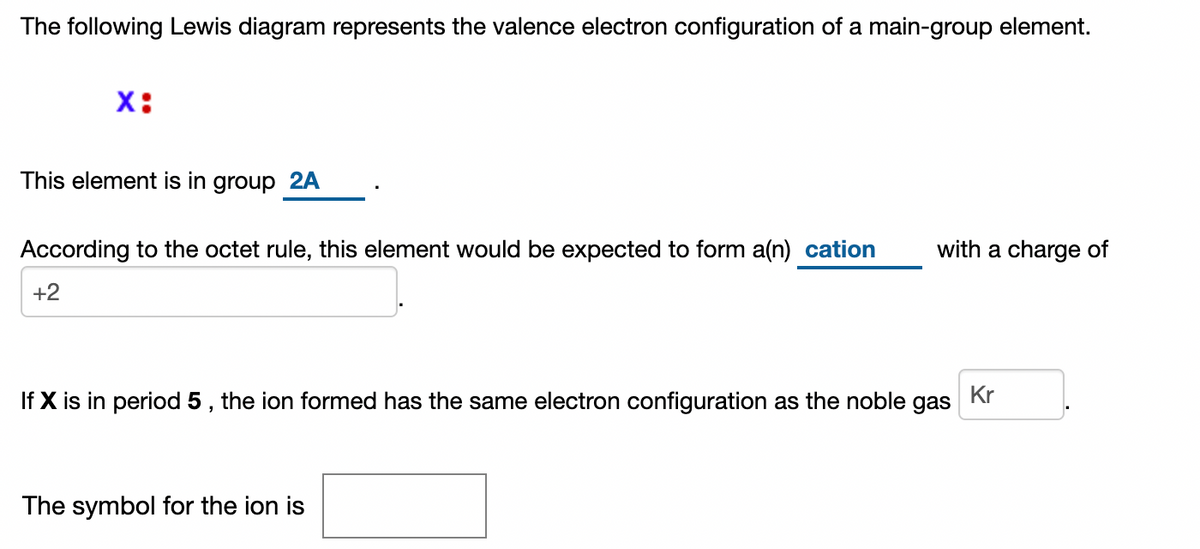
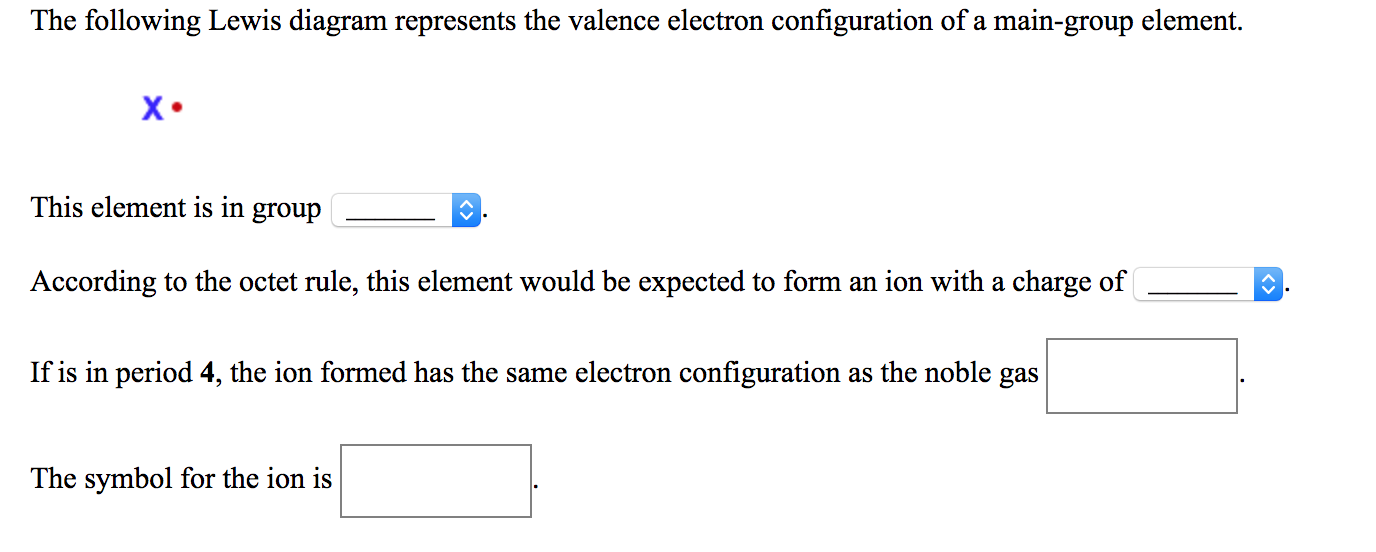
The following Lewis diagram represents the valence electron configuration of a main-group element. -B This element is in group According to the octet rule, this element would be expected to form a (n)with a charge of If X is in period 5, the ion formed has the same electron configuration as the noble gas The symbol for the ion is. Question: The ... Transcribed image text: The following Lewis diagram represents the valence electron configuration of a main-group element. This element is in group 2A ...
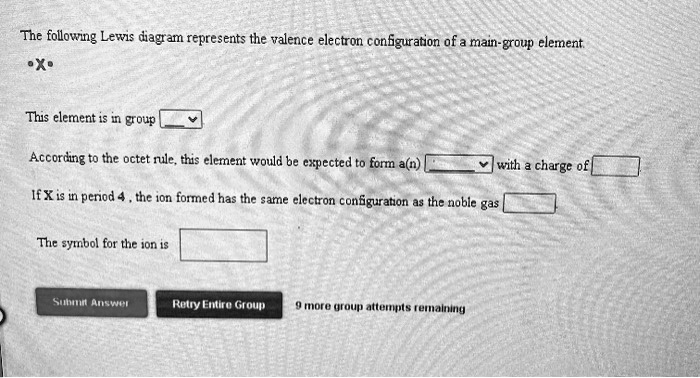
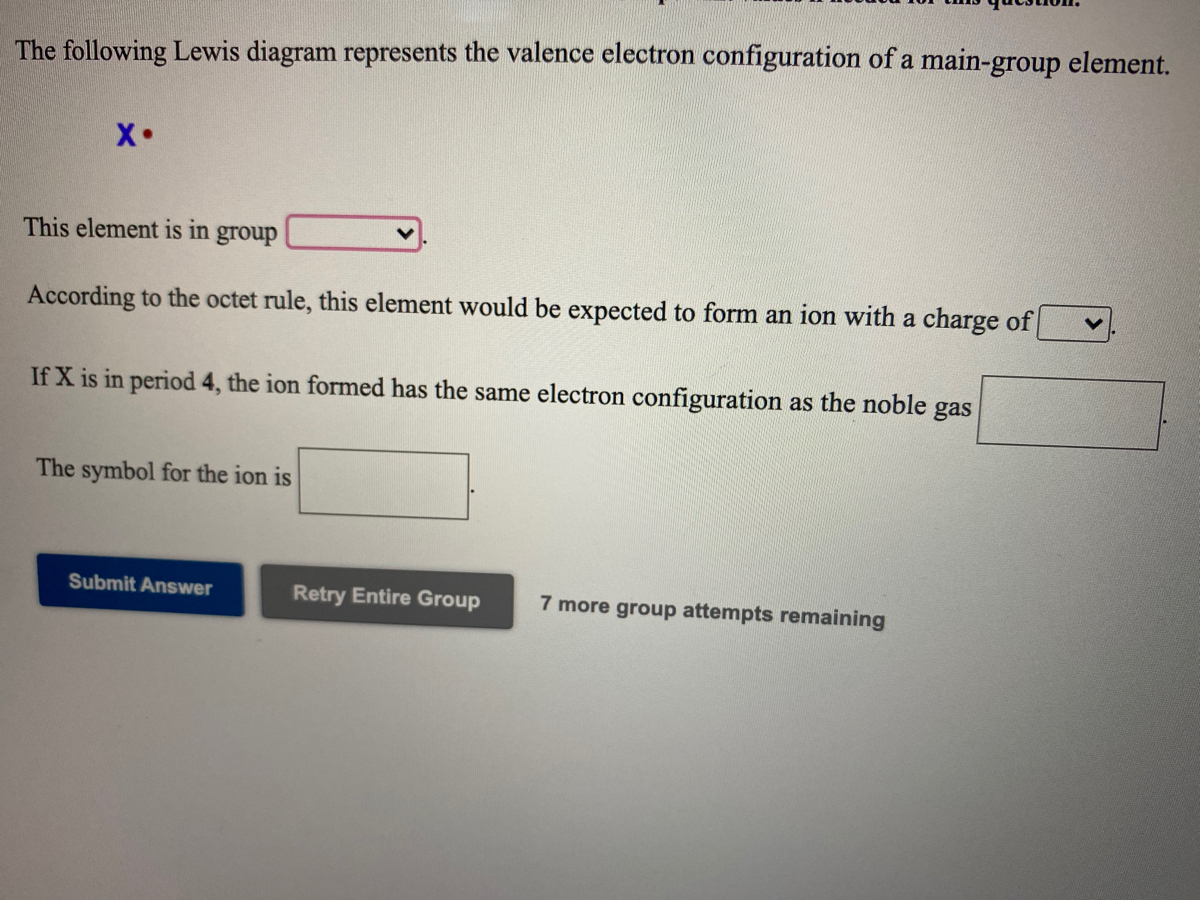
The following Lewis diagram represents the valence electron configuration of a main-group element. X This element is in group According to the octet rule, this element would be expected to form an ion with a charge of If X is in period 5, the ion formed has the same electron configuration as the noble gas The symbol for the ion is Submit Answer ...
The following lewis diagram represents the valence electron configuration of a main-group element.
Transcribed image text: The following Lewis diagram represents the valence electron configuration of a main-group element. -X This element is in group 2A ... Transcribed image text: The following Lewis diagram represents the valence electron configuration of a main-group element. If this element is in period 4, ... FREE Answer to The following Lewis diagram represents the valence electron configuration of a main-group element. X• The element...1 answer · Top answer: From the data this has 1 valence electrone 1 Valence electron has 'I' Group elementti Given 6th period element, and iie. 6th period, I Group element is ...
The following lewis diagram represents the valence electron configuration of a main-group element.. An element that has the valence electron configuration 6s26p6 belongs to which period and group? A) period 6; group 6A B) period 6; group 8A ... Draw the Lewis structure for NO2− including any valid resonance structures. Which of the following statements is TRUE? ... Which of the following signs on q and w represent a system that is doing ... Quantum number calculator. p . d f. This model takes the Pauli exclusion principle into account. Oct 20, 2016 · calculate the maximum and minimum number of electrons which may have magnetic quantum number m=+1 and spin quantum number s=+1/2in Cr - Chemistry - Atomic Structure and Nuclear Chemistry Learn what angular momentum is, principles behind this scientific phenomenon, the exact … Chemistry Q&A Library The following Lewis diagram represents the valence electron configuration of a main-group element. X- The element in period 5 that has this valence electron congfiguration is The following Lewis diagram represents the valence electron configuration of a main-group element. Electron affinity / configuration; Electronegativity ; Hardness; Heat capacity / of fusion / of vaporization; Ionization energy; Melting point; Oxidation state; Speed of sound; Thermal conductivity / expansion coefficient; Vapor pressure; Category; Chemistry Portal; A metalloid is a type of chemical element which has a preponderance of properties in between, or that are a mixture of, those of ...
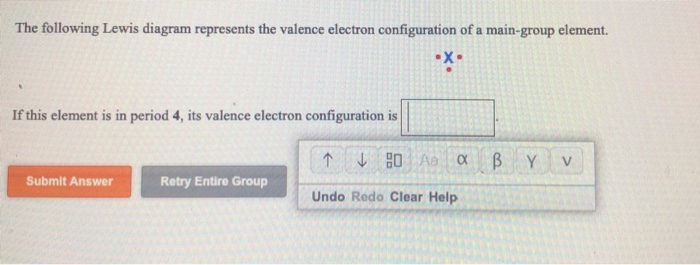
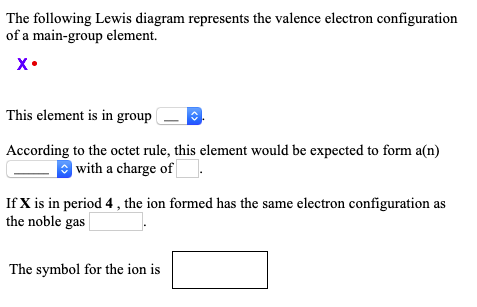
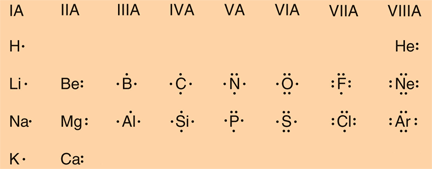
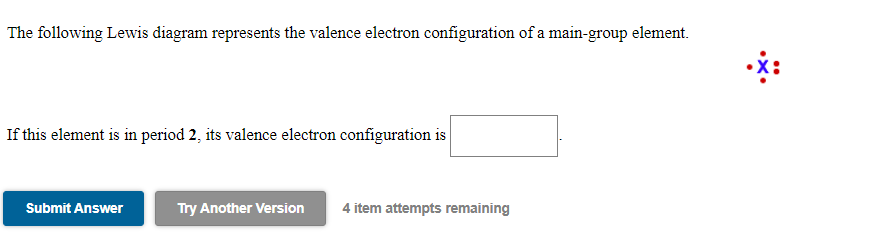
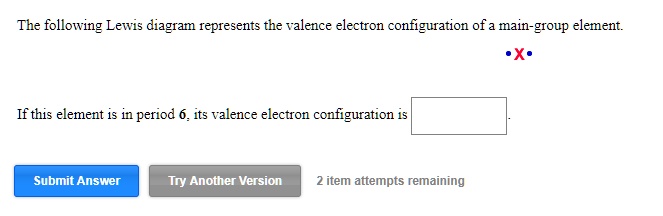
Classify each element as an alkali metal, alkaline earth metal, halogen, or noble gas. 7 - halogen. 1 - alkali metal. 8 - noble gas. 2 - alkaline earth metal. 2 - alkaline earth metal. Predict the charge of the ion formed by each element and write the electron configuration of the ion. S. The following Lewis diagram represents the valence electron configuration of a main-group element. If this element is in period 5, its valence electron configuration is The following Lewis diagram represents the valence electron configuration of a main-group element. CX The element in period 4 that has this valene electron congfiguration is. Transcribed image text: The following Lewis diagram represents the valence electron configuration of a main-group element. This element is in group 1A ... Transcribed image text: The following Lewis diagram represents the valence electron configuration of a main-group element. If this element is in period 6, ...
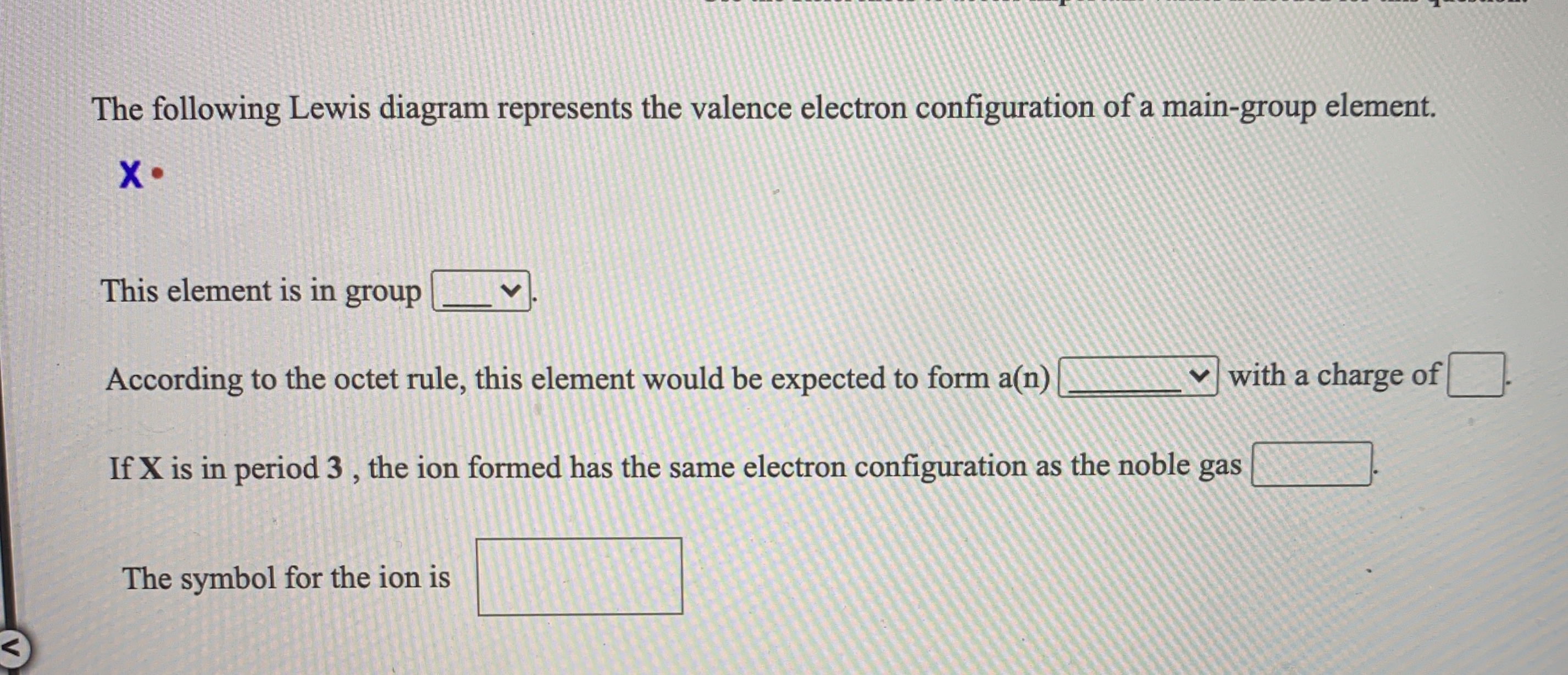
The following Lewis diagram represents the valence electron configuration of a main-group element EX. Show transcribed image text ... The following Lewis diagram represents the valence electron configuration of a main-group element. X• This element is in group According to the octet rule, this element would be expected to form a(n) v with a charge of If X is in period 3 , the ion formed has the same electron configuration as the noble gas The symbol for the ion is <> Question: The following Lewis diagram represents the valence electron configuration of a main-group element. .: The element in period 4 that has this valence electron congfiguration is 1A BA H 2A ЗА 4A SA SA 7A Не Li Be B C N O F Ne Na Mg 38 48 58 6B 7B 8B B2B Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb sr | Ý ... The following Lewis diagram represents the valence electron configuration of a main-group element. This element is in group According to the octet rule, this element would be expected to form an ion with a charge of If X is in period 3, the ion formed has the same electron configuration as the noble gas The symbol for the ion is Submit Answer ...
Valence shell electron pair repulsion theory is a simple way of rationalising the shapes of many compounds in which a main group element is surrounded by ligands. CH4 Lewis Structure Molecular Geometry Bond Angle geometryofmolecules. AX 4 ,AX 3 E and AX 2 E 2 Configurations. Use the VSEPR model to predict the molecular geometry of (a) O. •One can predict the shape of a molecule by finding …
A core-abbreviated electron configuration (right) replaces the core electrons with the noble gas symbol whose configuration matches the core electron configuration of the other element. Similarly, the abbreviated configuration of lithium can be represented as [He]2 s 1 , where [He] represents the configuration of the helium atom, which is identical to that of the filled inner shell of lithium.
Solved The Following Lewis Diagram Represents The Valence Electron Confguration Of Main Group Element Xe This Element Is In Group According To The Octet Rule This Element Would Be Expected To Form A N Wth
The Electron Configuration Video Lessons. Concept: Concept: Example: Problem: The following Lewis diagram represents the valence electron configuration of a main-group element. If this element is in period 6, its valence electron configuration is:
Transcribed image text: The following Lewis diagram represents the valence electron configuration of a main-group element, If this element is in period 4, ...

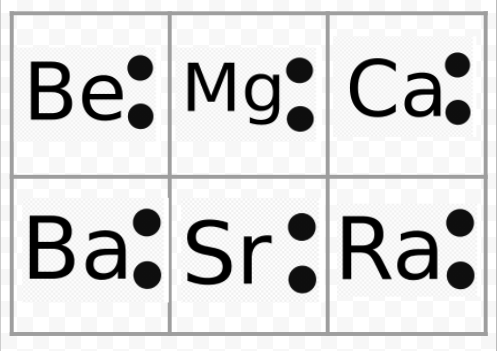
Draw An Electron Dot Diagram To Show The Formation Of Each Of The Following Compounds Magnesium Chloride H 1 C 6 Mg 12 Cl 17
Enter the email address you signed up with and we'll email you a reset link.
FREE Answer to The following Lewis diagram represents the valence electron configuration of a main-group element. X• The element...1 answer · Top answer: From the data this has 1 valence electrone 1 Valence electron has 'I' Group elementti Given 6th period element, and iie. 6th period, I Group element is ...
Transcribed image text: The following Lewis diagram represents the valence electron configuration of a main-group element. If this element is in period 4, ...
Transcribed image text: The following Lewis diagram represents the valence electron configuration of a main-group element. -X This element is in group 2A ...
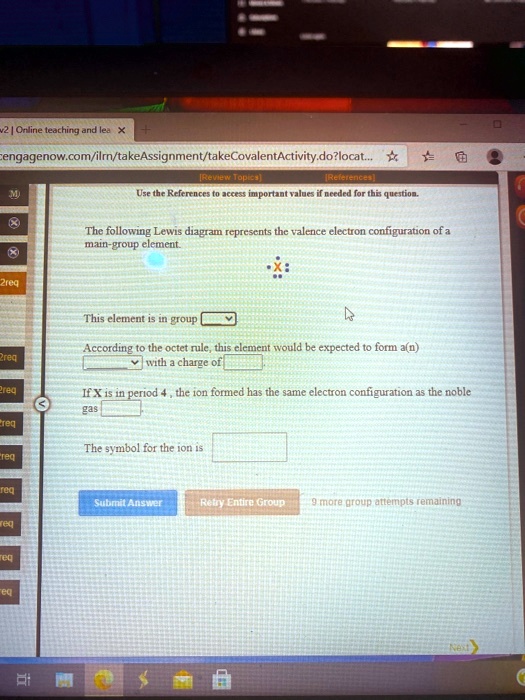
Solved Odine Leachina And Engagenow Com Ilrn Takeassignment Takecovalentactivity Dozlocat Hopisa Msehe Eeemencer Jc Tt Mmportant Tl0r Mncrurd Quuatioa The Following Lewis Diagram Rcprescnts Thc Valence Clectron Configuration Of A Mn Eicud Elemeut
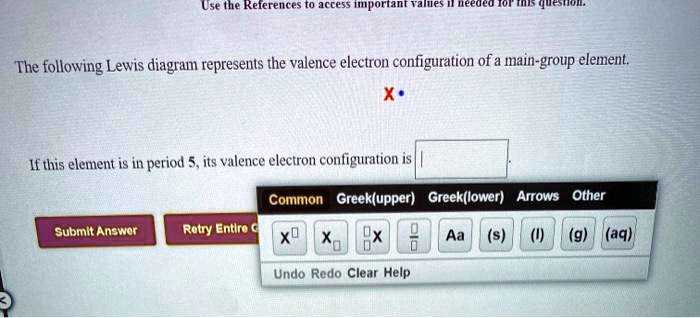
Solved Usethe Relerences E Md The Following Lewis Diagram Represents The Valence Electron Configuration Of A Main Group Element L This Element Is In Period Its Valence Electron Configuration Is Common Greek Upper

The Following Lewis Diagram Represents The Valence Electron Configuration Of A Main Group Element X The Element Homeworklib

Solved This The 5 Element Is In Group Following Lewis The Ion Forned Has This Diagram Represents Element The Same Would 4 Valence H Electron As The A N Configuration Of A Noble Gas







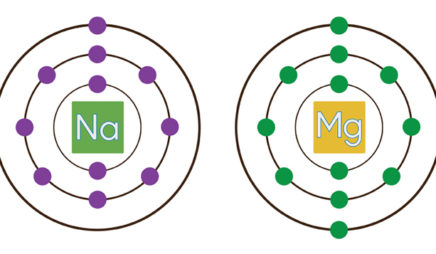





0 Response to "37 the following lewis diagram represents the valence electron configuration of a main-group element."
Post a Comment