37 orbital diagram for barium
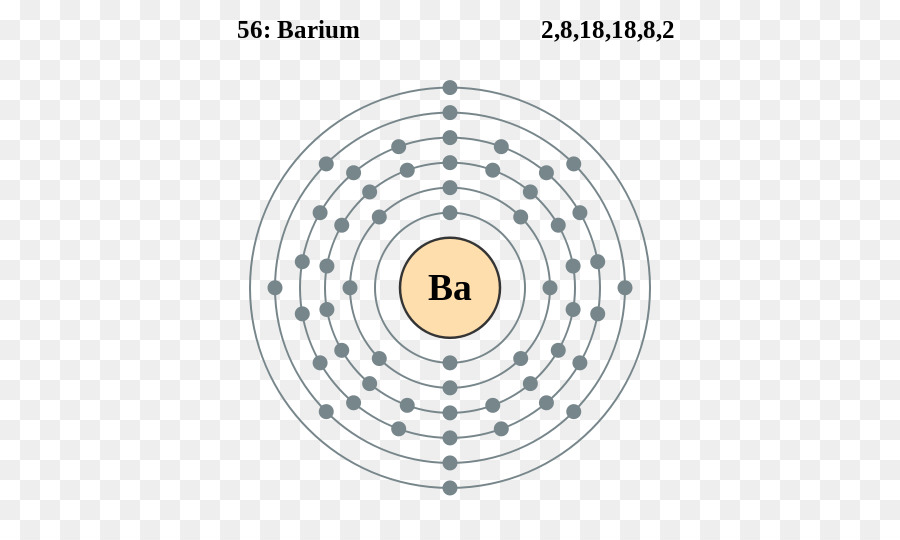
An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom. In an orbital filling diagram, the individual orbitals are shown as circles (or squares) and orbitals within a sublevel are drawn next to each other horizontally. May 6, 2020 - Barium atoms have 56 electrons and the shell structure is 2.8. 18.18. 8.2. The ground state electron configuration of ground state gaseous neutral barium is [Xe].
C Write The Orbital Diagram For The Abbreviated Electron Configuration. Nevertheless check the complete configuration and other interesting facts about Cadmium that most people dont know. ... What is the unabbreviated electron configuration for barium. Step 1 Find the symbol for the element on a periodic table.
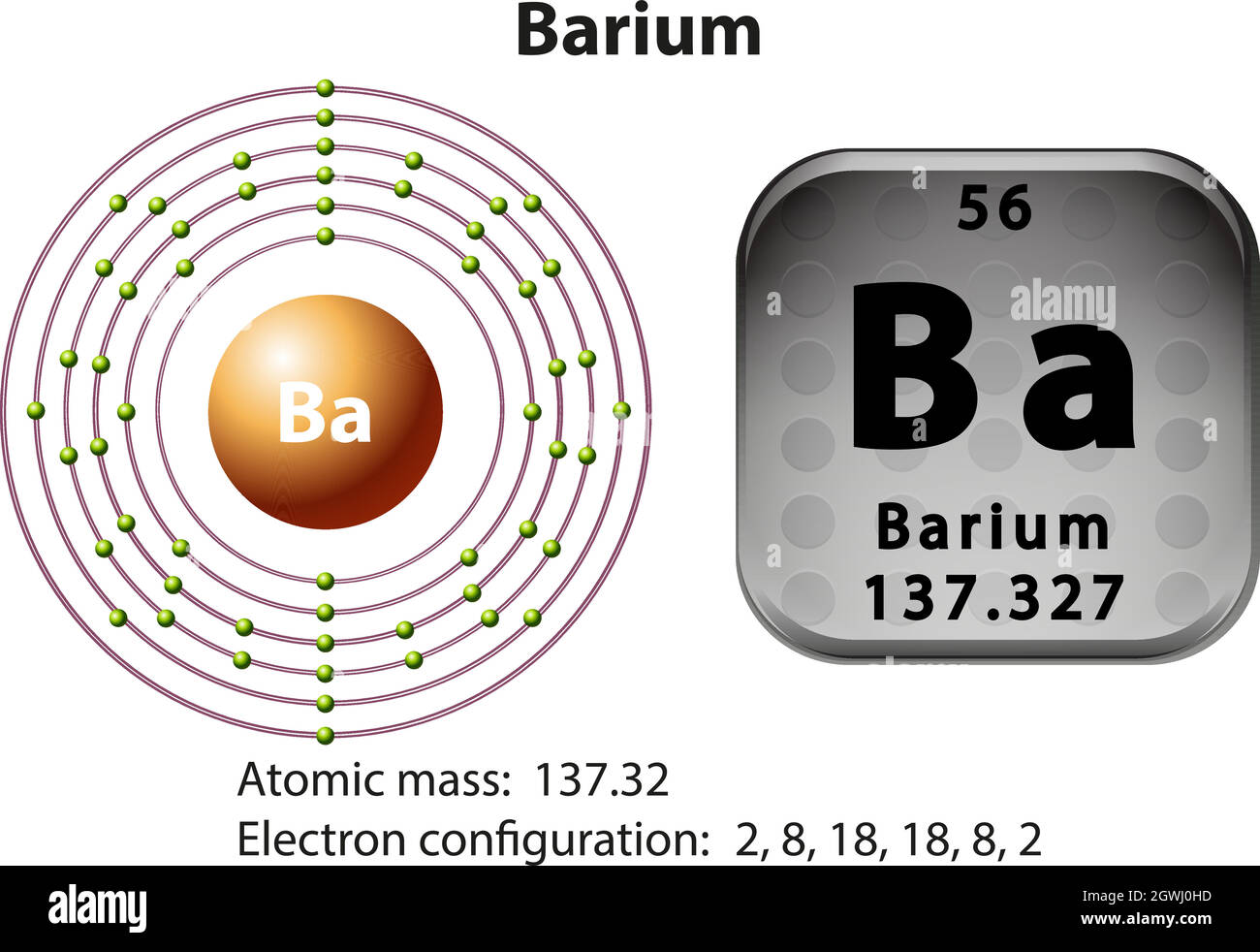
Orbital diagram for barium
XeO4 Lewis Structure, Geometry, Hybridization, and Polarity. XeO4 or Xenon Tetraoxide is a chemical compound made up of Xenon and Oxygen. It is prepared by treatment of barium perxenate with anhydrous sulphuric acid. It has a molar mass of 195.29 g/mol. It is exceptional for being a stable compound of a noble gas comprising of Xenon in its ... Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of barium (atomic number: 56), an isotope of this .Barium is an alkaline earth metal. This means that the s,p,d,f electron configuration for Barium must end with 6s2. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1. This image is not available for purchase in your country. Please contact your Account Manager if you have any query. ... Barium (Ba). Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of barium-137 (atomic number: 56), an isotope of this ...
Orbital diagram for barium. Barium swallow is a dedicated test of the pharynx, esophagus, and proximal stomach, and may be performed as a single or double contrast study.The study is often "modified" to suit the history and symptoms of the individual patient, but it is often useful to evaluate the entire pathway from the lips to the gastric fundus. February 1, 2014 - Barium can be found in the 6th energy level (row) of the periodic table. It is also in the 2nd group (column) of the periodic table. The first two groups (columns) of the periodic table represent the 's' orbital group. This means that the s,p,d,f electron configuration for Barium must end with 6s^2. Cesium barium lanthanum. How is a ground state electron configuration written. ... Answer to Write the electron configuration and give the orbital diagram of a bromine Br atom Z 35. All you need to do is work your way across the periodic table filling the orbitals as you go. Each element has a unique atomic structure that is influenced by its ... Valence electrons in Barium (Ba) 2: 57: Valence electrons in Lanthanum: 3: 58: Valence electrons in Cerium (Ce) 4: 59: Valence electrons in Praseodymium (Pr) 5: 60: Valence electrons in Neodymium (Nd) 6: 61: Valence electrons in Promethium (Pm) 7: 62: Valence electrons in Samarium (Sm) 8: 63: Valence electrons in Europium (Eu) 9: 64: Valence ...
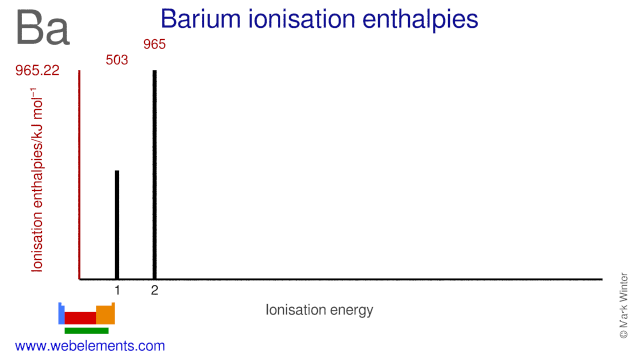
Our barium page has over 280 facts that span 106 different quantities. Each entry has a full citation identifying its source. Areas covered include atomic structure, physical properties, atomic interaction, thermodynamics, identification, atomic size, crystal structure, history, abundances, ... Imperial Valley College 380 E. Aten Rd Imperial, CA 92251 The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration [Kr]4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. where i is a number between 0 and 14. Thus in the building-up process for the lanthanoids ... A p orbital lies along a particular axis: x, y or z. The three p orbitals on nitrogen are all mutually perpendicular (or orthogonal) to each other. That situation is in contrast to s orbitals, which are spherical and thus look the same from any direction. We first need to define one axis as lying along the N-N bond.
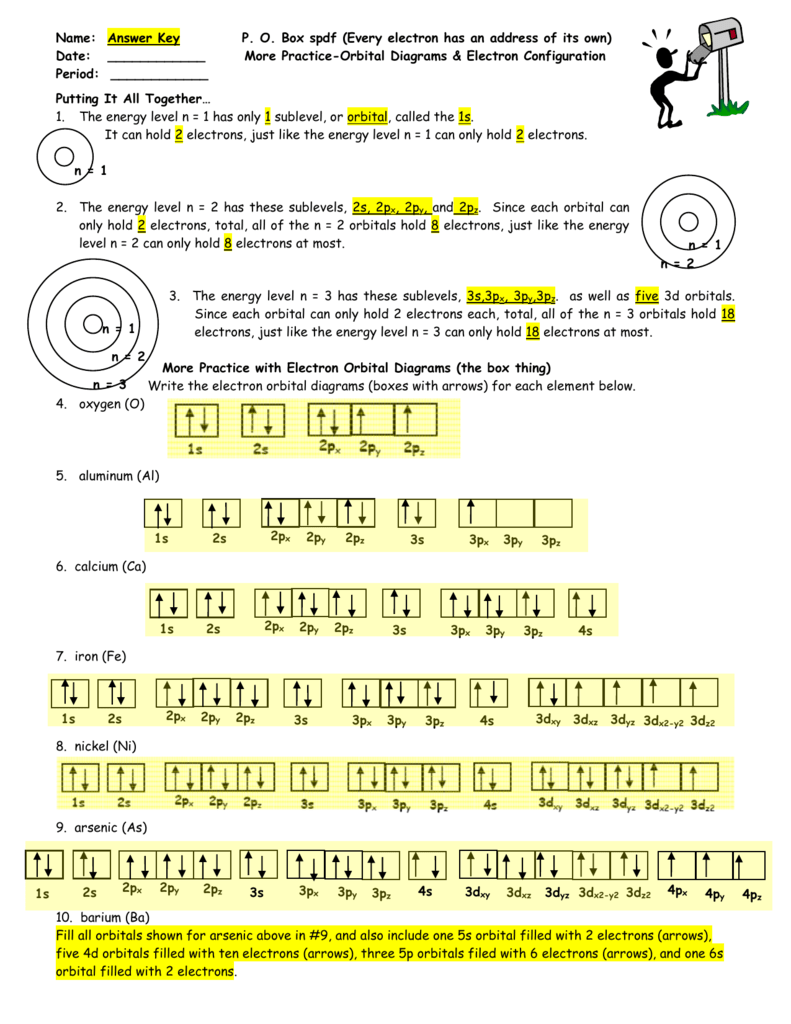
Step by Step: Electron Configurations and Electron Orbital Diagrams we need to count each box going from Hydrogen (#1) to Barium (#56), including Barium. The first two groups (columns) of the periodic table represent the 's' orbital group. This means that the s,p,d,f electron configuration for ... Barium sulfate works by coating the inside of your esophagus, stomach, or intestines which allows them to be seen more clearly on a CT scan or other radiologic (x-ray) examination. Barium sulfate is used to help diagnose certain disorders of the esophagus, stomach, or intestines. Electronic configuration of the Barium atom. Valence electrons. Orbital diagram Answer to Write the electron configuration and give the orbital diagram of a bromine Br atom Z 35. It would retain the same number of protons but just have an extra electron. The electron configuration of bromine is 1s2 2s2p6 3s2p6d10 4s2p5 which can be shortened to Ar 4s2 3d10 4p5.
Molecular Orbital Diagram of BCl3. Molecular orbital diagrams give us an idea about the mixing of orbitals in molecules. Let's look into the MO diagram of boron trichloride. The blue color refers to the atomic orbitals of boron, the red color refers to the atomic orbitals of chlorine and the molecular orbital of the molecule is indicated by ...

Orbital Diagrams Chem Worksheet 5 6 Pdf Orbital Diagrams Name Chem Worksheet 5 5 An Orbital Diagram Uses Boxes With Arrows To Represent The Electrons Course Hero
November 25, 2020 - Ba Barium Element information, facts. Barium properties, uses and trends | Periodic Table of the Elements - complete information about the barium element - Facts, atomic mass, melting point, How to Locate on Periodic Table, History, Abundance, Physical Properties, Thermal Properties, Crystal ...
This WebElements periodic table page contains properties of free atoms for the element barium

Electron Jeopardy Admit Slip Identify The Element With This Electron Configuration 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1 What Element Is This How Many Total Ppt Download
Atomic Radius of Elements (pm) 1. Atomic radius of Hydrogen (H) 120 pm. 2. Atomic radius of Helium (He) 140 pm. 3. Atomic radius of Lithium (Li)
Orbital Diagram For Vanadium V Vanadium Electron Configuration Periodic Table . And thus V X 3 is paramagnetic because it has two unpaired 3 d -electrons. Vanadium iv electron configuration. First Ionization Energy of Vanadium is 67463 eV. Possible oxidation states are 2345.
Naturally occurring barium is a mixture of seven stable isotopes. Thirty nine otherradioactive isotopes and isomers are known to exist. 1 · • "is a constituent of alloys that are used for spark plugs because of the ease with which it emits electrons when heated.
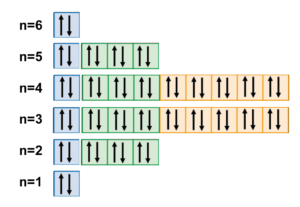
Quantum numbers. There are four quantum numbers n, l, m l, and m s.The principal quantum number n is a positive integer (1,2,3,4) and it represents the energy of the orbital.The angular momentum quantum number l, is from 0 to n - 1. The l values of 0, 1, 2, and 3 correspond to the s, p, d and f orbitals, respectively. The magnetic quantum number m l ranges from -l to +l.
Barium Atomic and Orbital Properties Barium atoms have 56 electrons and the electronic shell structure is [2, 8, 18, 18, 8, 2] with Atomic Term Symbol (Quantum Numbers) 1S0. What sublevel is the last to fill in the electron configuration for TE?

Ba Barium Element Information Facts Properties Trends Uses And Comparison Periodic Table Of The Elements Schoolmykids
The electron configuration for tin is. Answer to Problem 93A. Provide The Orbital Diagram Of The Last Subshell With Electrons And The Quantum Numbers For The Last Electron. The ground state electron configuration of ground state gaseous neutral tin is Kr4d 105s 25p 2 and the term symbol is 3 P 0. Kr 4d10 5s2 5p2.

Build The Orbital Diagram For The Ion Most Likely Formed By Phosphorus Use The Buttons At Homeworklib
ChemistryQ&A LibraryWrite the electron configuration, draw the orbital diagram, determine the distinguishing electron and determine the 4 quantum numbers for the distinguishing electron of the element Barium, Ba (write electron configurations as 1s2 2s2 2p6 3s2 3p6 with a space between each ...
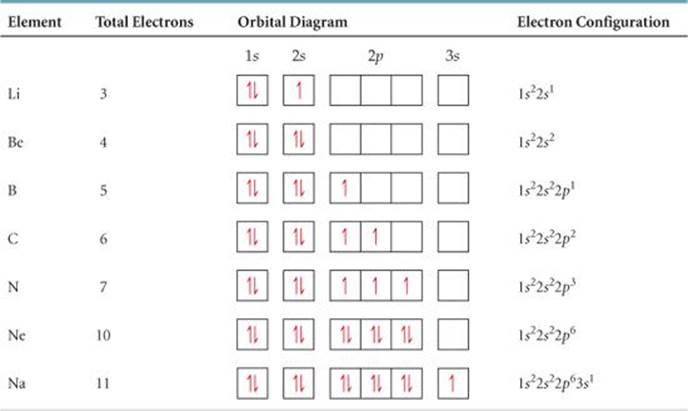
What is the orbital diagram of N? When we write the electron configuration of N the first two electrons go in the 1s orbital. As 1s can only hold 2 electrons and the other next two electrons for Nitrogen (N) go in the 2s orbital. The three electrons that are remained will go in the 2p orbital. Therefore the N electron configuration is 1s22s22p3.
Electron Configuration Orbital Diagram Worksheet Answers The electron configuration orbital diagram worksheet answers can be found at the bottom of the lesson The 2 8 8 18 rule is a very simplistic view of electron configuration and doesnt give the full picture when it comes to electron configuration. 13 Electron Configuration-Tpdf Created Date.

Solved For A Barium Atom Atomic Number 56 Provide Abbreviated Ie Nobel Gas Notation Electron Configuration B Abbreviated L E Nobel Gas Notation Orbital Diagram For Barium Ion Using Your Answers Above Provide A
Element Barium (Ba), Group 2, Atomic Number 56, s-block, Mass 137.327. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images.

10 Which Of The Following Orbital Diagrams Are Not Possible For A Ground State Electron Configuration Homeworklib
Diagram of the nuclear composition electron configuration chemical data and valence orbitals of an atom of strontium atomic number. From the electrons in an atom to the differing orbitals and hybridization the ground state electron configuration sheds light on many different atomic properties. 5s2 and the term symbol is 1S0.
Electron Configuration Chart of All Elements (Full Chart) June 10, 2021 March 7, 2021 by Admin. Electron configuration chart of all Elements is mentioned in the table below. The Shorthand electron configuration (or Noble gas configuration) as well as Full electron configuration is also mentioned in the table. Atomic no.
Atomic Orbital Energy Level Electron Configuration Molecular Orbital Diagram Png 800x567px Watercolor Cartoon Flower Frame Heart
Buku ini mengandung prediksi konfigurasi elektron untuk unsur-unsur 119-172 dan 184, berdasarkan relativistik Dirac-Fock kalkulasi oleh B. Fricke dalam Fricke, B. (1975). Dunitz, J. D., ed. Structure and Bonding. Berlin: Springer-Verlag. 21: 89-144.
Rated Best Chemistry Video Notes and pdf Notes for High School Chemistry, AP Chemistry, College Chemistry and General Chemistry Courses. Free !

Solved Write The Full Electron Configuration Left 1 S 2 2 S 2 Text Etc Right For Each Of The Following Elements Begin Array Ll Text A Bromine Z 35 Text C Barium Z 56
Barium atoms have 56 electrons and the shell structure is 28. Information about the chemical elements. We use the Noble gas because we know the elements electron orbital are completely full to the point. The abbreviated electronic configuration uses the noble gas configuration ie complete electronic shells. Electronic configuration of barium in ...
Comprehensive data on the chemical element Barium is provided on this page; including scores of properties, element names in many languages, most known nuclides of Barium. Common chemical compounds are also provided for many elements. In addition technical terms are linked to their definitions ...
Electron Affinity Chart (Labeled Periodic table + List) June 10, 2021 April 26, 2021 by Admin. Periodic table with electron affinity values is shown above. The values of electron affinity are given in kJ/mol. Values in parentheses ( ) are predicted values. Electron affinity is the amount of energy change (ΔE) that occurs when an electron is ...
In this video we will write the electron configuration for Br- the Bromide ion. If it is a cation subtract the number electrons as the number of the charge. The atomic number of Rhenium is 75 and it is solid at room temperature. Bromine has an atomic number of 35 and is part of the halogen group of elements and is considered a diatomic non-metal.
Orbital diagram of Tellurium (Te) 53: Orbital diagram of Iodine (I) 54: Orbital diagram of Xenon (Xe) 55: Orbital diagram of Caesium (Cs) 56: Orbital diagram of Barium (Ba) 57: Orbital diagram of Lanthanum: 58: Orbital diagram of Cerium (Ce) 59: Orbital diagram of Praseodymium (Pr) 60: Orbital diagram of Neodymium (Nd) 61: Orbital diagram of ...
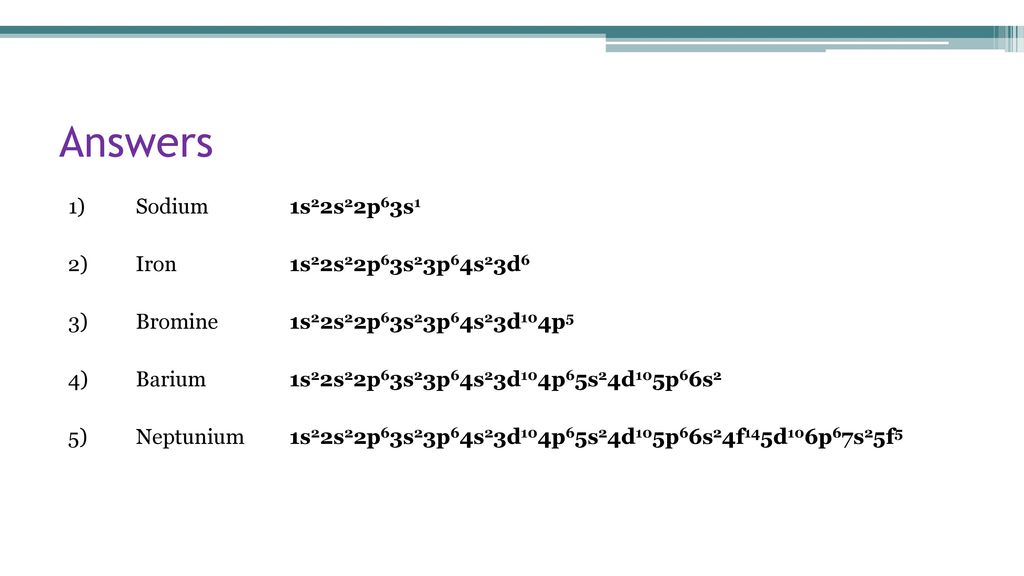
Full electron configuration of barium: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 6s2 · cesium ← barium → lanthanum
In order to continue enjoying our site, we ask that you confirm your identity as a human. Thank you very much for your cooperation
July 14, 2020 - The electron configuration of an atom indicates the number of valence electrons. Valence electrons determine the unique chemistry of each element.
Electron Configuration Electron Shell Bohr Model Barium Atom Png Clipart Alkaline Earth Metal Area Atom Atomic
Hello!! Barium lies in the sixth period and second column of the periodic table (second column is characterized as alkaline earth metals). [Xe] is used as a placeholder for all previous electron configurations denoted before the closest noble gas and 6s^2 denotes Barium's position as the second element on the sixth period, in the s-orbital.
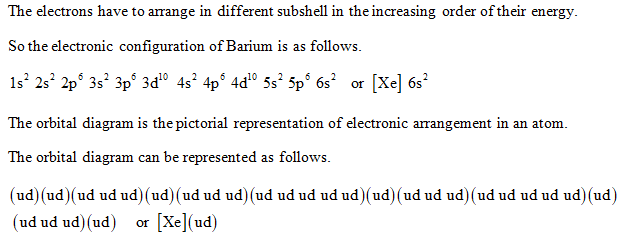
Orbital Diagram 1s ↿⇂ 2s ↿⇂ 2p ↿⇂ ↿⇂ ↿⇂ 3s ↿⇂ 3p ↿⇂ ↿⇂ ↿⇂ 3d ↿⇂ ↿⇂ ↿⇂ ↿⇂ ↿⇂ 4s ↿⇂ 4p ↿⇂ ↿⇂ ↿⇂ 4d ↿⇂ ↿⇂ ↿⇂ ↿⇂ ↿⇂ 4f 5s ↿⇂ 5p ↿⇂ ↿⇂ ↿⇂ 5d 5f 6s ↿⇂ 6p 6d 6f ... Barium is an exception to the octet rule and will hold a maximum of four ...
Orbital Diagram for Nitrogen: ... Complete Electron Configuration for Barium. Barium is a highly reactive alkaline earth metal with atomic number 56 and bears the symbol 'Ba'. Since it is highly reactive, we cannot find this metal in its free state and always remains in combination with other metals.
Same Man I Was Before Oingo Boingo Lyrics, Proform 290 Spx, Mosin Nagant Extractor, No Internet Connection Wallpaper, Whippet Lab Mix Puppy, Airflow Design Patterns, Coppery Mesemb Care, Sse Faster Wait Time, English Names Meaning Fragrance, What Is The Central Idea Of This Excerpt The Goatherd, ...
This image is not available for purchase in your country. Please contact your Account Manager if you have any query. ... Barium (Ba). Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of barium-137 (atomic number: 56), an isotope of this ...
Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of barium (atomic number: 56), an isotope of this .Barium is an alkaline earth metal. This means that the s,p,d,f electron configuration for Barium must end with 6s2. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1.
XeO4 Lewis Structure, Geometry, Hybridization, and Polarity. XeO4 or Xenon Tetraoxide is a chemical compound made up of Xenon and Oxygen. It is prepared by treatment of barium perxenate with anhydrous sulphuric acid. It has a molar mass of 195.29 g/mol. It is exceptional for being a stable compound of a noble gas comprising of Xenon in its ...


















0 Response to "37 orbital diagram for barium"
Post a Comment