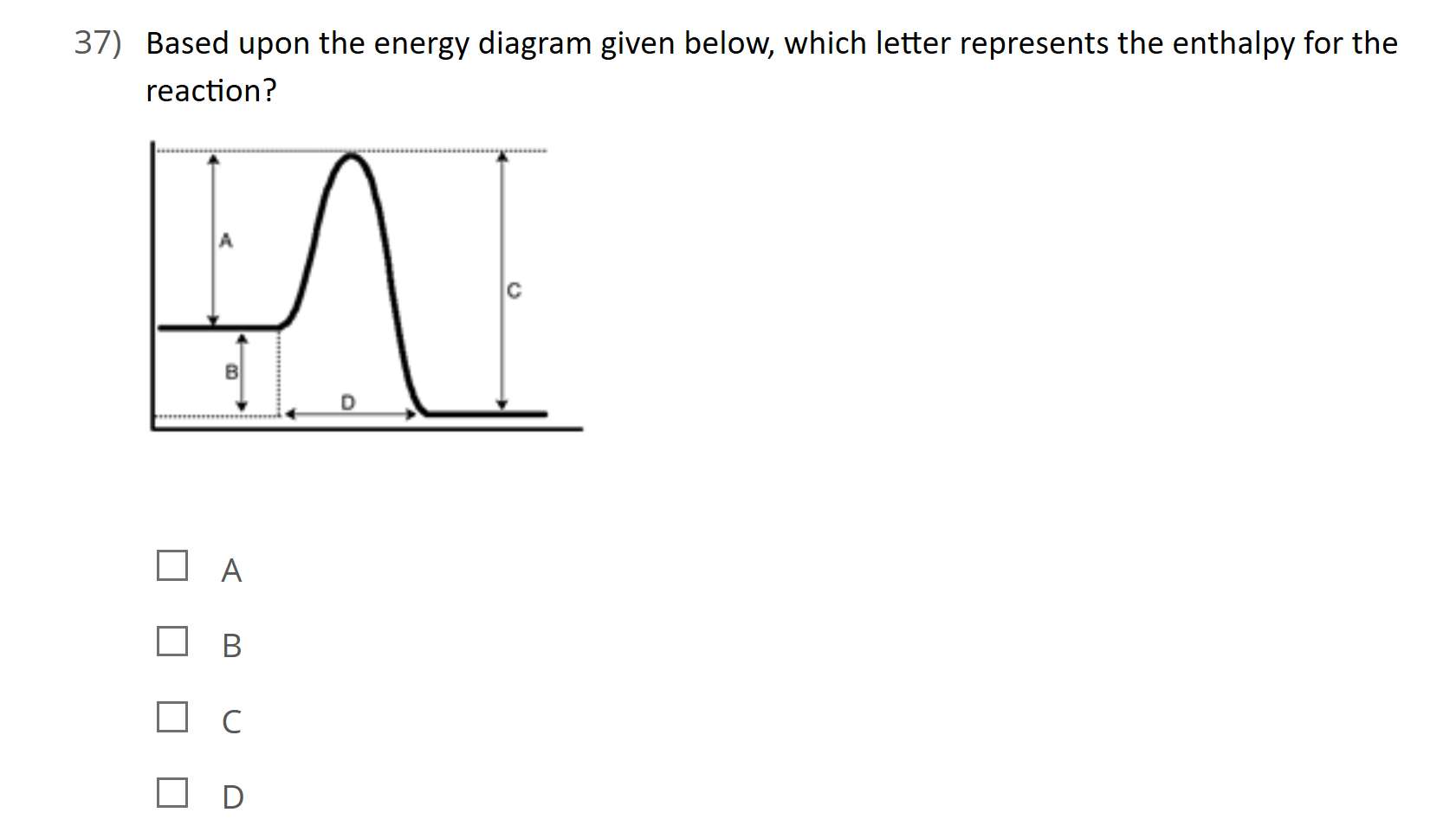
37 this diagram would represent the enthalpy change in which of the following
This diagram would represent the enthalpy changes in which of the following? boiling liquid hot pack cold pack melting solid.1 answer · Top answer: Answer is: hot pack. This is exothermic reaction (energy or heat is released), because reactants have higher energy than products of reaction. Hot ... >> The enthalpy change for whi... Question. The enthalpy change for which of the following processes represents the enthalpy of formation of AgCl :-A. A g + ... Important Diagrams > Real Life Applications > Common Misconceptions > Best Reference on Internet > Problem solving tips >
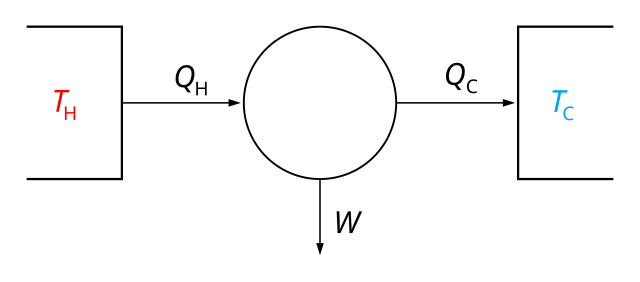
2 The following equation shows the formation of ammonia. –N2(g) + –H2(g) NH3(g) The graph shows how the free-energy change for this reaction varies with temperature ... Calculate the enthalpy change for this reaction. ... The diagram shows a non-rechargeable cell that can be used to power electronic devices.

This diagram would represent the enthalpy change in which of the following
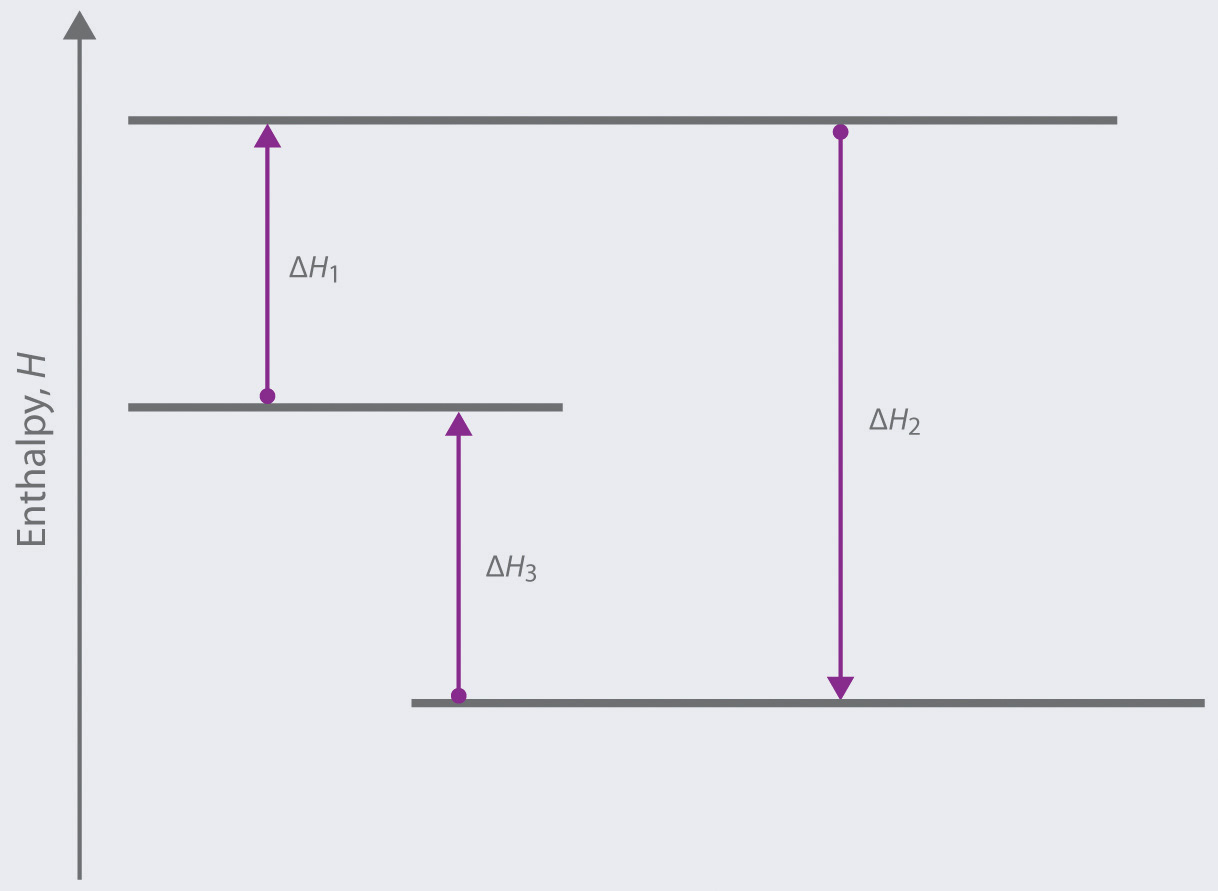
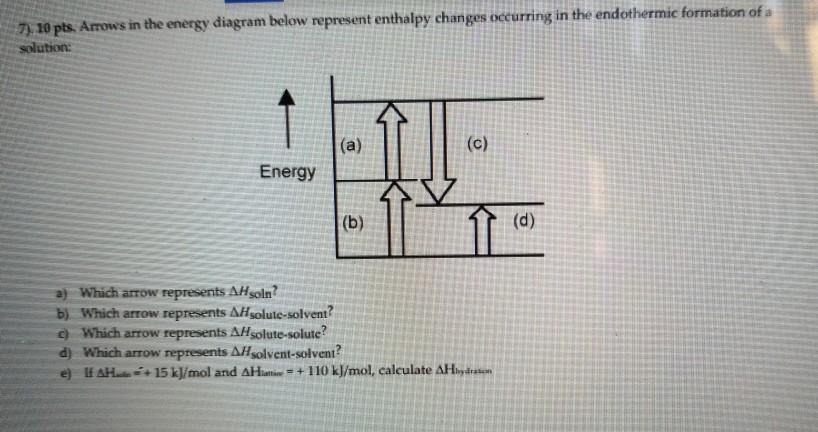
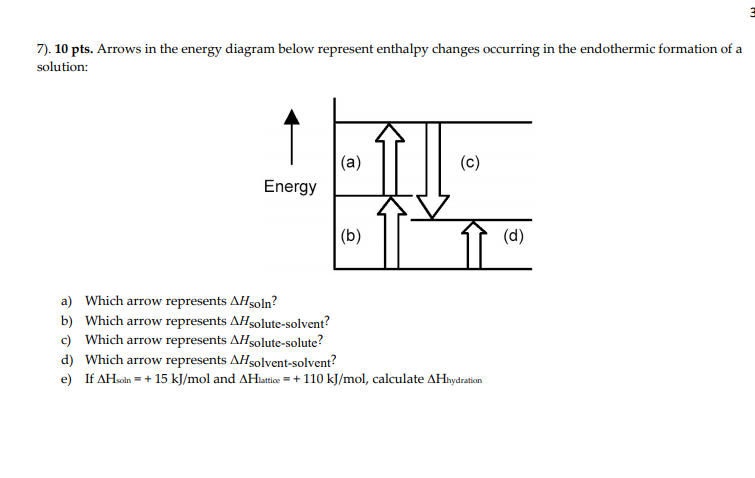
Chemistry. Chemistry questions and answers. Arrows in the energy diagram below represent enthalpy changes occurring in the formation of a solution: | (a) (c) Energy (b) (d) Which arrow represents Asoln? O A arrow (a) OB, arrow (b) O Carrow (d) D. arrow (c) Question: Arrows in the energy diagram below represent enthalpy changes occurring in the ... 9 The following energy cycle represents the enthalpy changes in the formation of carbon dioxide from its constituent elements in their standard states. What substances are present at level Y in this diagram? A C(g) + 2O(g) B C(g) + O 2(g) C C(s) + O 2(g) D CO 2(g) energy level Y 0 ∆H f V_ 2 This diagram would represent the enthalpy changes in which of the following? boiling liquid hot pack cold pack melting solid.
This diagram would represent the enthalpy change in which of the following. Will mark as Brainliest. This diagram would represent the enthalpy changes in which of the following? cold pack hot pack melting solid boiling liquid. (ii) Suggest why this enthalpy change cannot be measured directly. [1] b)( Enthalpy changes of combustion can often be measured directly. The equation for the reaction which represents the enthalpy change of combustion (∆Hc)fthanol o e is as follows. C 2H5OH(I) + 3O 2(g) 2CO 2(g) + 3H 2O(I) 10:33How to draw enthalpy diagrams from a chemical reaction and a dH value.Table of Contents:00:14 - Learning ...26 Feb 2016 · Uploaded by kiefersci 3 Mar 2018 — This diagram would represent the enthalpy changes in which of the following? boiling liquid hot pack cold pack melting solid · Answer · Did this ...1 answer · 51 votes: Answer is: hot pack.This is exothermic reaction (energy or heat is released), because reactants have higher energy than products of reaction. Hot ...
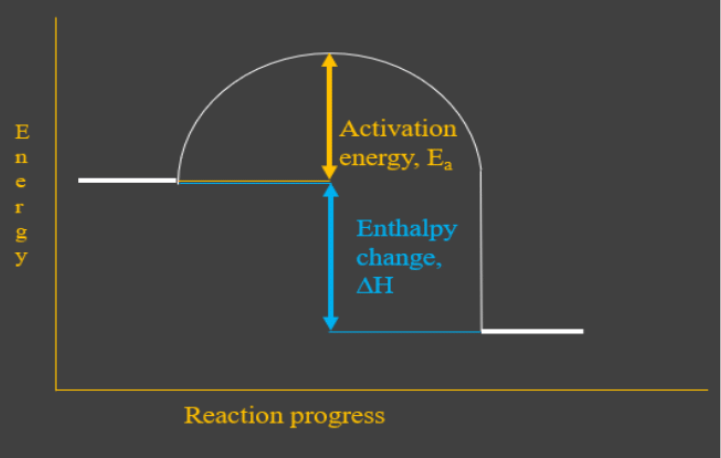
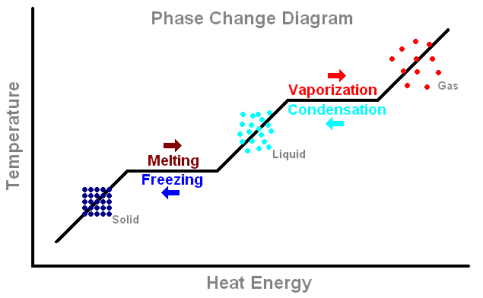
When a substance changes from solid to liquid, liquid to gas or solid to gas, there are specific enthalpies involved in these changes. The enthalpy (or latent heat) of melting describes the transition from solid to liquid (the reverse is minus this value and called the enthalpy of fusion), the enthalpy of vaporization describes the transition from liquid to gas (and the opposite is ... This diagram would represent the enthalpy changes in which of the following? boiling liquid hot pack cold pack melting solid. Q. "Heat change when 1 mole of gaseous atom is formed from its element at standard states" is the definition for _____. This diagram would represent the enthalpy change in which of the following? liquid water freezing A 10 g gold coin is heated from 25°C to 50°C (CAu is 0.13 J/g-°C).
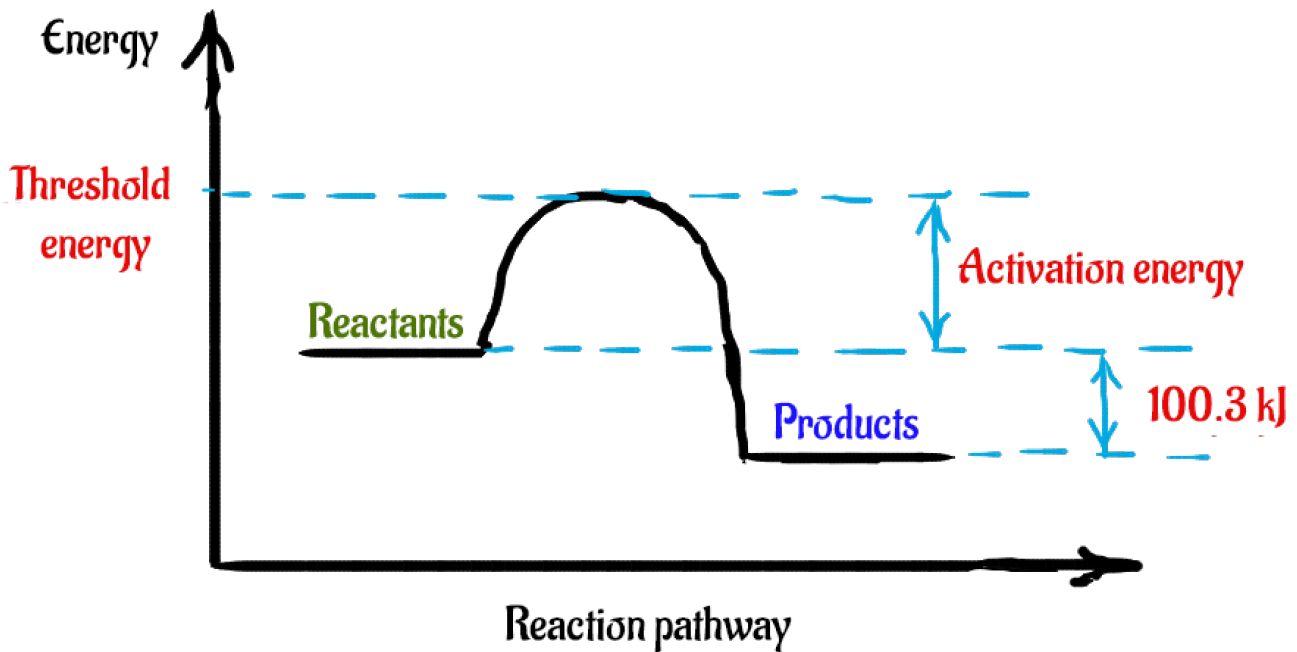
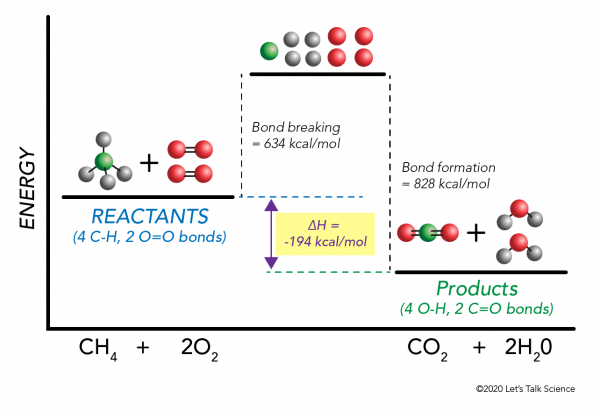
There are two key points about enthalpy that are related to chemistry: - Most chemical reactions occur at constant pressure and volume, so in chemistry—and in this course—we will refer to enthalpy as it relates to the thermal energy of the system (not work). This diagram would represent the enthalpy changes in which of the following? boiling liquid hot pack cold pack melting solid. 9 The following energy cycle represents the enthalpy changes in the formation of carbon dioxide from its constituent elements in their standard states. What substances are present at level Y in this diagram? A C(g) + 2O(g) B C(g) + O 2(g) C C(s) + O 2(g) D CO 2(g) energy level Y 0 ∆H f V_ 2 Chemistry. Chemistry questions and answers. Arrows in the energy diagram below represent enthalpy changes occurring in the formation of a solution: | (a) (c) Energy (b) (d) Which arrow represents Asoln? O A arrow (a) OB, arrow (b) O Carrow (d) D. arrow (c) Question: Arrows in the energy diagram below represent enthalpy changes occurring in the ...













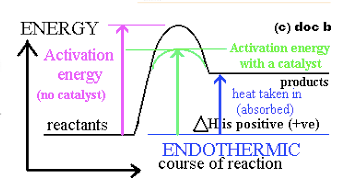


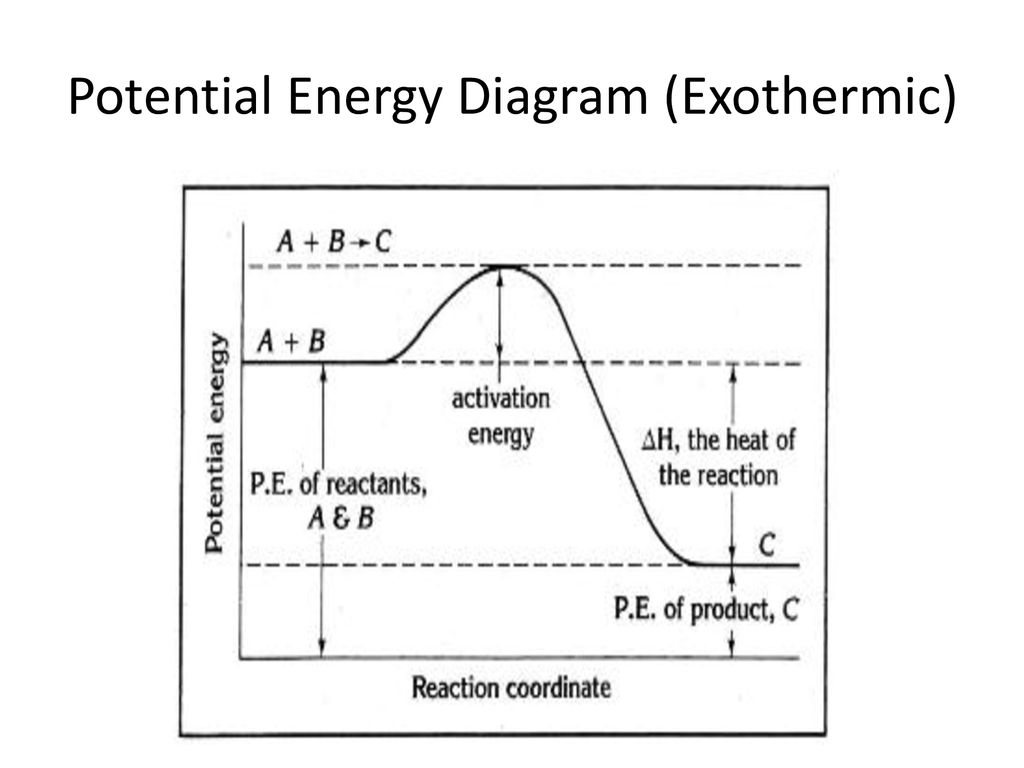
0 Response to "37 this diagram would represent the enthalpy change in which of the following"
Post a Comment