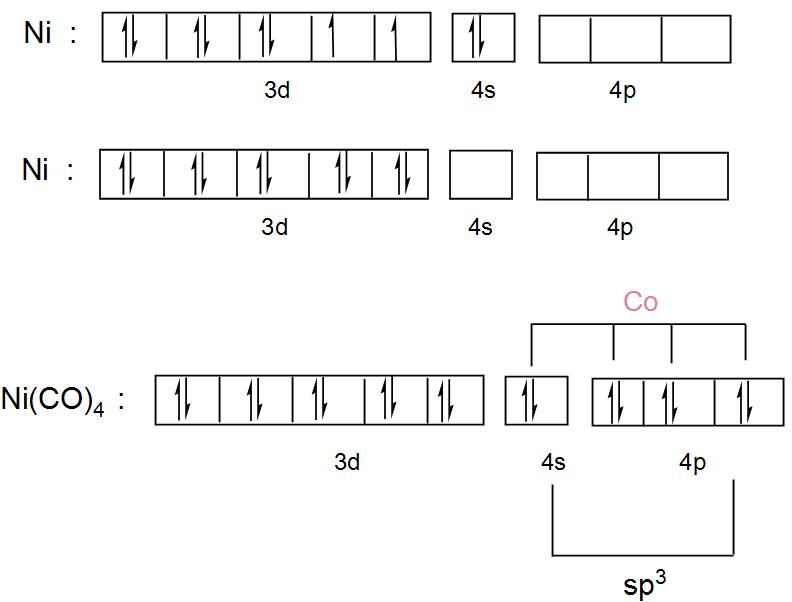
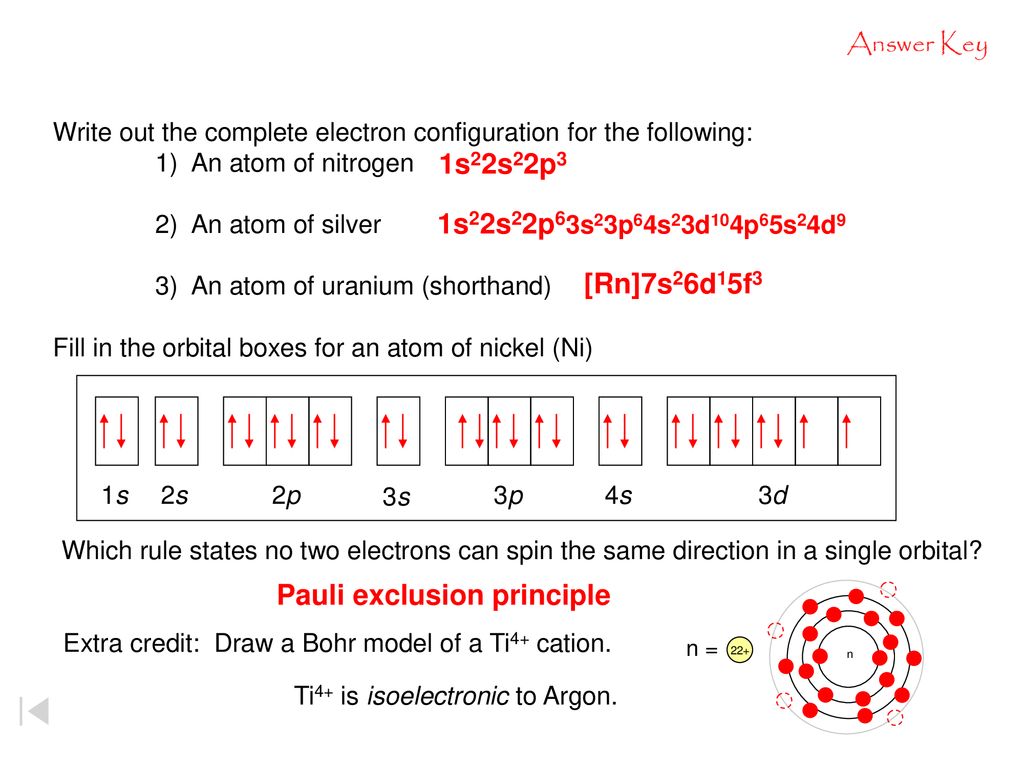
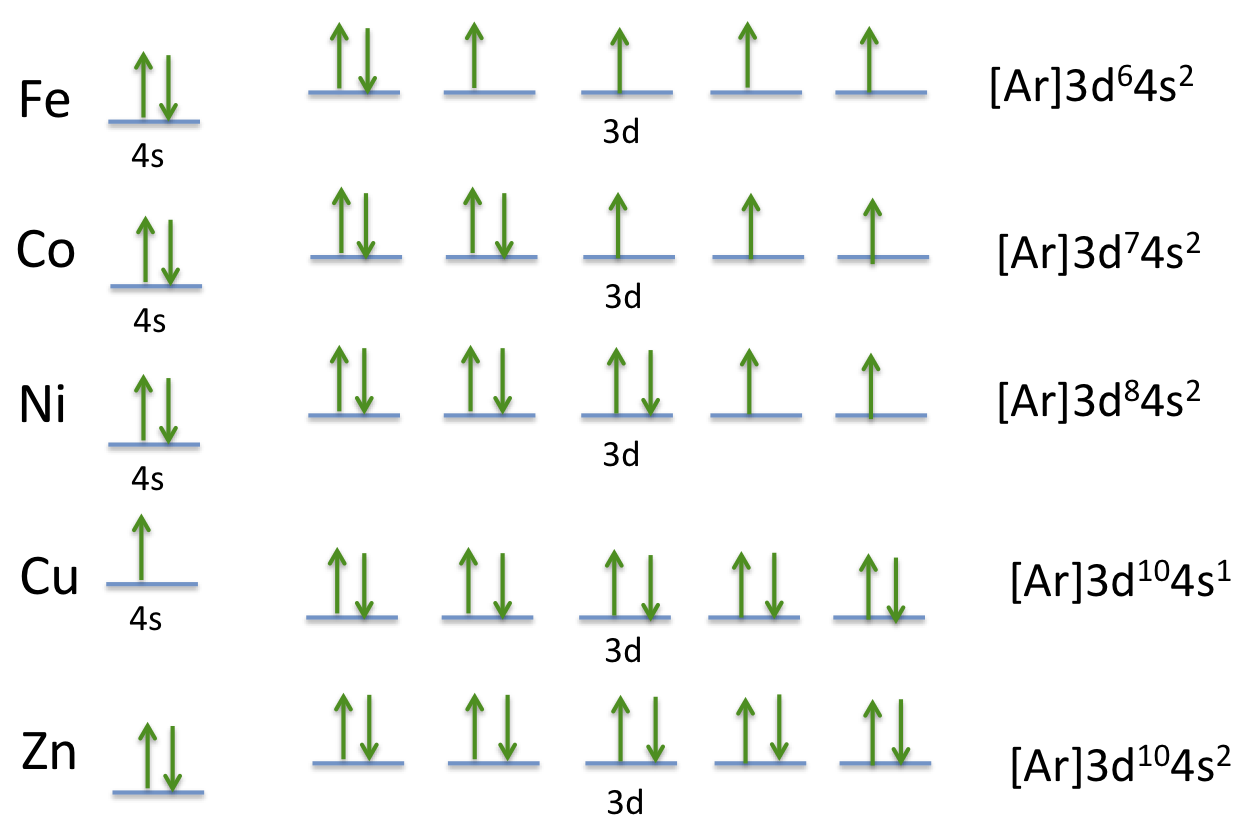
38 construct the orbital diagram for ni.
We're being asked to construct the orbital diagram for Ni.For that, we first need to determine the electron configuration of Ni.. Recall that for a neutral element, Atomic number = # of protons = # of electrons. The atomic number of Ni is 28 and since it's a neutral element, this means Ni has 28 electrons. Procedure for Constructing Molecular Orbital Diagrams Based on Hybrid Orbitals 1. Begin with the Lewis structure. 2. Decide how many orbitals each atom needs to make its sigma bonds and to hold its non-bonding electrons. Draw the atomic and hybrid orbitals on on side of the page. 3. For each sigma bond, take a hybrid (or atomic) orbital from ...
Construct the orbital diagram for ni. Construct the orbital diagram for ni. The atomic number of ni is 28 and since its a neutral element this means ni has 28 electrons. The oxides are then treated with an acid that reacts with the iron not the nickel. Nickels atomic number is 28 so we need to arrange 28 electrons. ...

Construct the orbital diagram for ni.
Their blank d -splitting diagrams within the realm of crystal field theory are: [Ni(CN)4]2−: The d orbitals fill with 8 electrons, then, with a low spin configuration. You can see that an even number of d orbitals will get filled ( dyz,dxz,dz2,dxy) with an even number of 3d electrons. This gives rise to a diamagnetic configuration, as expected. Refer to the explanation. The electron configuration of manganese, atomic number 25, is "1s"^2"2"^2"2p"^6"3s"^2"3p"^6"3d"^5"4s"^2". The diagram below represents the electron configuration as an orbital diagram. This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n...
Construct the orbital diagram for ni.. Orbital diagrams are a visual way to show where the electrons are located within an atom. Orbital diagrams must follow 3 rules: The Aufbau principle, the Pau... Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Transcribed image text: Construct the orbital diagram for nickel. Answer Bank 111 Energy. Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom Answer (1 of 4): Nickel is atomic number 28; therefore, it has 28 electrons in its orbitals. The filling rules are as follows: 1. Aufbau Principle: Lowest energy levels fill first. 2. Pauli Exclusion Principle: Only 2 electrons per orbital, they must have opposite spin. 3. Hund's Rule: Given sev...
Transcribed Image Textfrom this Question. Construct the orbital diagram for nickel 1 11 1 4p 1 11 14 1 3d 1 4s Answer Bank 1 1 1 3p 1 1 1L 3s 1 1 1L 2p 1 2s 1s Energy Construct the orbital diagram of the F ion Зр Answer Bank 3s 1 2p 2.s 1s Energy. Construct the orbital diagram for as. Given the valence electron orbital level diagram a. Given the valence electron orbital level diagram a. Expert answer 100 14 ratings 1s 2s 2p 3s 3p 3d 4s 4p 4d 4f 5s 5p 5d 5f 6s 6p 6d 7s 7p ok starting from 1s you have to go diagonally view the full answer. Problem: Construct the orbital diagram for Ni. FREE Expert Solution. Ni → atomic # 28 → 28 electrons. Ni will pass through 1s, 2s, 2p, 3s, 3p, 4s2, 3d. Following Aufbau principle (fill lowest energy first) and Hund's rule (half-filled first before totally filled) 82% (205 ratings) An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box, line, or circle, is drawn to represent each orbital in the electron configuration. (using the Aufau Principle to order the orbitals and hence the boxes, lines or circles, as shown below) 1s. →. 2s.
(ii) Complete the diagram to show the splitting of the d orbital energy levels in an octahedral complex ion. energy (iii) On the axes below, sketch the shapes of one d orbital from the lower energy level and one d orbital from the higher energy level. y lower energy level z x y z x higher energy level [4] Physical Properties Density 8, 9 g/cm3 Atomic Volume 6. thickness : 8.9 g/cm3: atomic Volume : 6.6 cm3/mole: atomic Radius : 1.24 angstroms • MO diagrams can be built from group orbitals and central atom orbitals by considering orbital symmetries and energies. • The symmetry of group orbitals is determined by reducing a reducible representation of the orbitals in question. This approach is used only when the group orbitals are not obvious by inspection. orbital diagram and that would force us to fill in the bonding sigma MO and the anti-bonding sigma-star MO. What we gain in the bonding sigma MO, we lose in the anti-bonding sigma-star MO. There is no advantage for two helium atoms to join together in a molecule, and so they remain as isolated atoms (note
The orbital diagram for nickel is as follows: 1s2 2s2 2p6 3s2 3p6 4s2 3d8. In all of the cases, both up and down arrows are filled, with the exception of the 3d shell, where the last two are up ...
DOWNLOAD Wiring Diagrams Free. Close DOWNLOAD. Construct The Orbital Diagram For Ni. More Details . 76 Plymouth Duster Dome Light Wiring Diagram. More Details . 2003 Envoy 4.2 Liter Cooling Fan Wiring Diagram. More Details . Jvc Kd-g230 Wiring Diagram. More Details . Warwick Corvette Wiring Diagram.

Atomic Orbital Energy Level Electron Configuration Molecular Orbital Diagram Energy Angle White Text Png Pngwing
Question: Construct the orbital diagram for Ni. Construct the orbital diagram for Ni. see more. Best answer. Atomic orbital diagrams are also known as electron-in-a-box diagrams. These are simplified diagrams of how electrons are arranged within the orbitals for a. Answer to Construct the orbital diagram for Ni. Start by adding the appropriate ...
Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
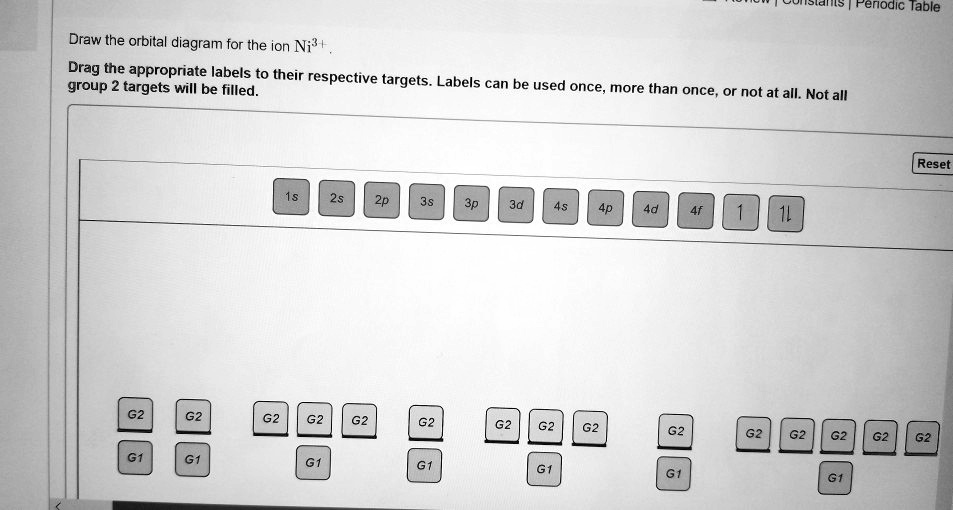
Solved Feriodic Lable Draw The Orbital Diagram For The Ion Ni Drag The Appropriate Labels To Their Respective Group 2 Targets Will Be Filled Targets Labels Can Be Used Once More Than Once
Construct the orbital diagram for ni. Write the electron configuration full and in core notation for the following ions. Write the electron configuration full and in core notation. The oxides are then treated with an acid that reacts with the iron not the nickel. Construct the orbital diagram for ni. Start by adding the appropriate subshells ...
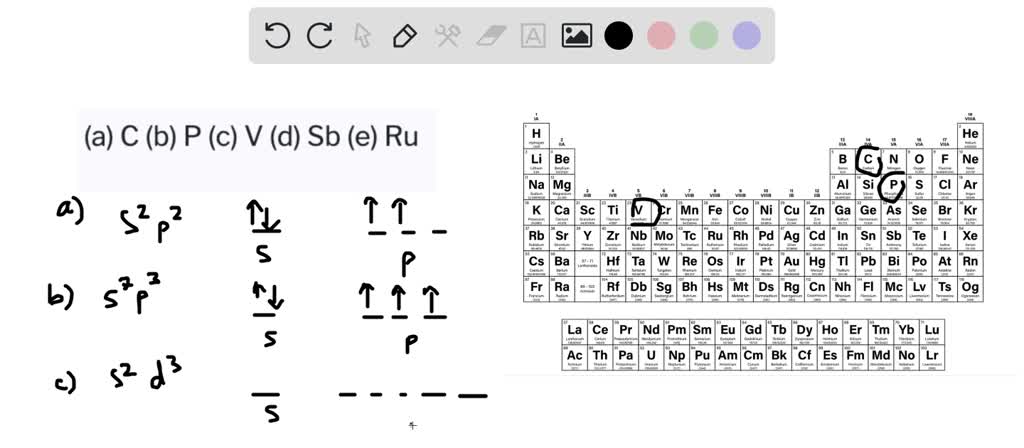
Solved Draw The Orbital Diagram For The Valence Shell Of Each Of The Following Atoms A C B P C V D Sb E Ru
construct construct the orbital diagram for ni molecular orbital diagram a molecular orbital diagram or mo diagram is a qualitative descriptive tool. image file c7ta k f8 tif. electron configurations and orbital diagrams key electron configurations and orbital diagrams key draw orbital diagrams for the following elements 1 phosphorus.
The atomic number of ni is 28 and since its a neutral element this means ni has 28 electrons. 1s2 2s2 2p6 3s2 3p6 4s2 3d8. Electron configurations and orbital diagrams key draw orbital diagrams for the following elements. The diagram not to scale summarises the energies of the orbitals up to the 4p level.
Construct the orbital diagram for ni. Construct the orbital diagram for ni. These are simplified diagrams of how electrons are arranged within the orbitals for a. The metal is produced by heating the ore in a blast furnace which replaces the sulfur with oxygen. Draw the orbital diagram for ion co 2.
Answer to Construct the orbital diagram for Ni. Start by adding the appropriate subshells. For example, carbon is in the 2p block.1. Describe the two differences between a 2p x orbital and a 3p y orbital. The 2px orbital lies on the x-axis. The 3py orbital lies on the y-axis and is larger than the 2px orbital. 2.
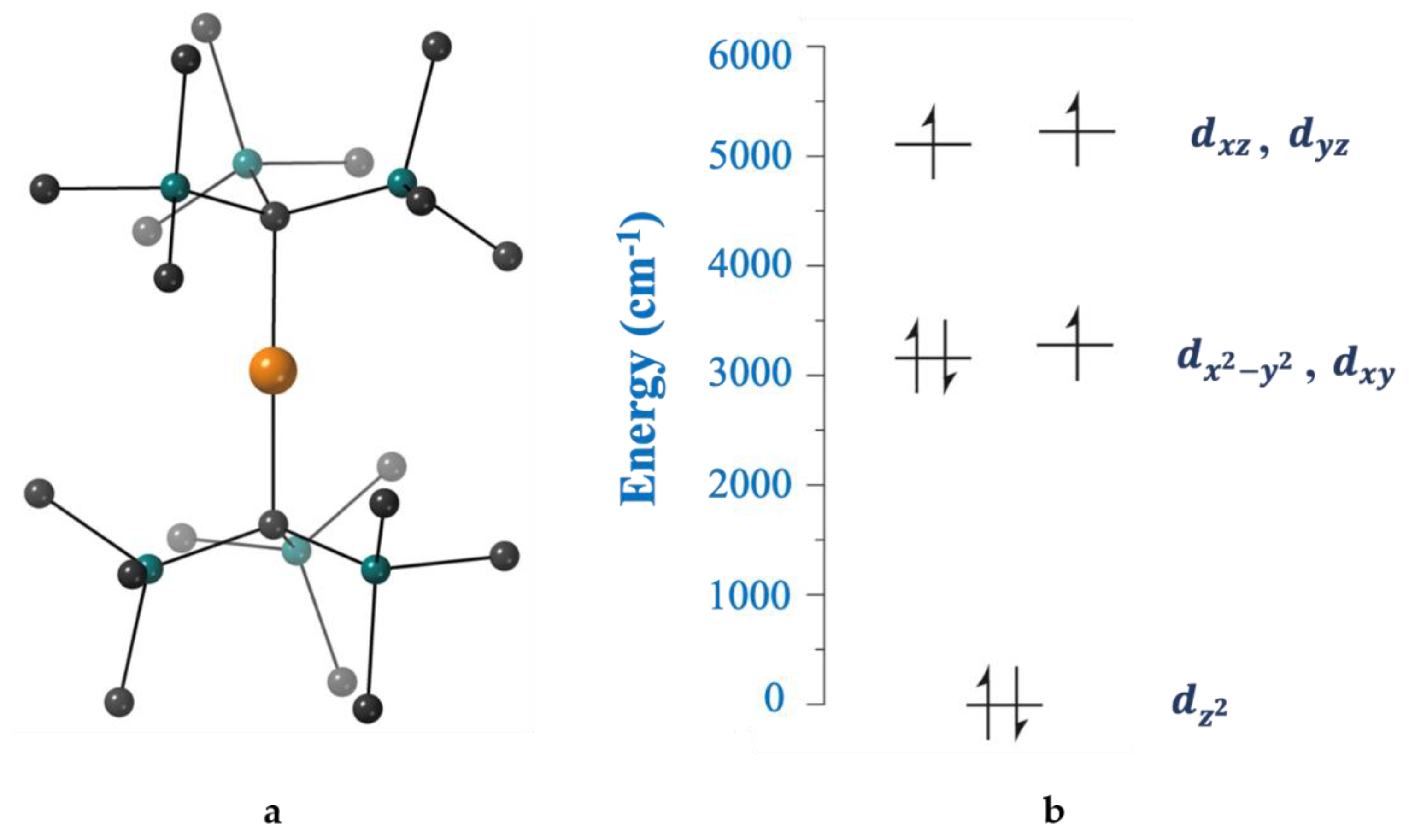
Inorganics Free Full Text Smart Ligands For Efficient 3d 4d And 5d Metal Single Molecule Magnets And Single Ion Magnets Html
Construct The Orbital Diagram For Ni. 03.06.2019 03.06.2019. 76 Plymouth Duster Dome Light Wiring Diagram ... 03.06.2019 03.06.2019. 2007 Suzuki Forenza Wiring Diagram For Keyless Entry. 03.06.2019 03.06.2019. Rochester Quadrajet Carburetor Vacuum Diagram. 03.06.2019 03.06.2019. Tls2-gd2 Wiring Diagram ... Vanadium Orbital Diagram; 92 Cadillac ...
This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n...
Refer to the explanation. The electron configuration of manganese, atomic number 25, is "1s"^2"2"^2"2p"^6"3s"^2"3p"^6"3d"^5"4s"^2". The diagram below represents the electron configuration as an orbital diagram.
Their blank d -splitting diagrams within the realm of crystal field theory are: [Ni(CN)4]2−: The d orbitals fill with 8 electrons, then, with a low spin configuration. You can see that an even number of d orbitals will get filled ( dyz,dxz,dz2,dxy) with an even number of 3d electrons. This gives rise to a diamagnetic configuration, as expected.

Single Crystal Growth And Effects Of Ni Doping On The Novel 12442 Type Iron Based Superconductor Rbca2fe4as4f2 Iopscience

Promotion Effect Of Metal Phosphides Towards Electrocatalytic And Photocatalytic Water Splitting Han 2021 Ecomat Wiley Online Library

6 Draw Orbital Energy Splitting Diagrams And Use The Spectrochemical Series To Show The Orbital Occupancy For Homeworklib
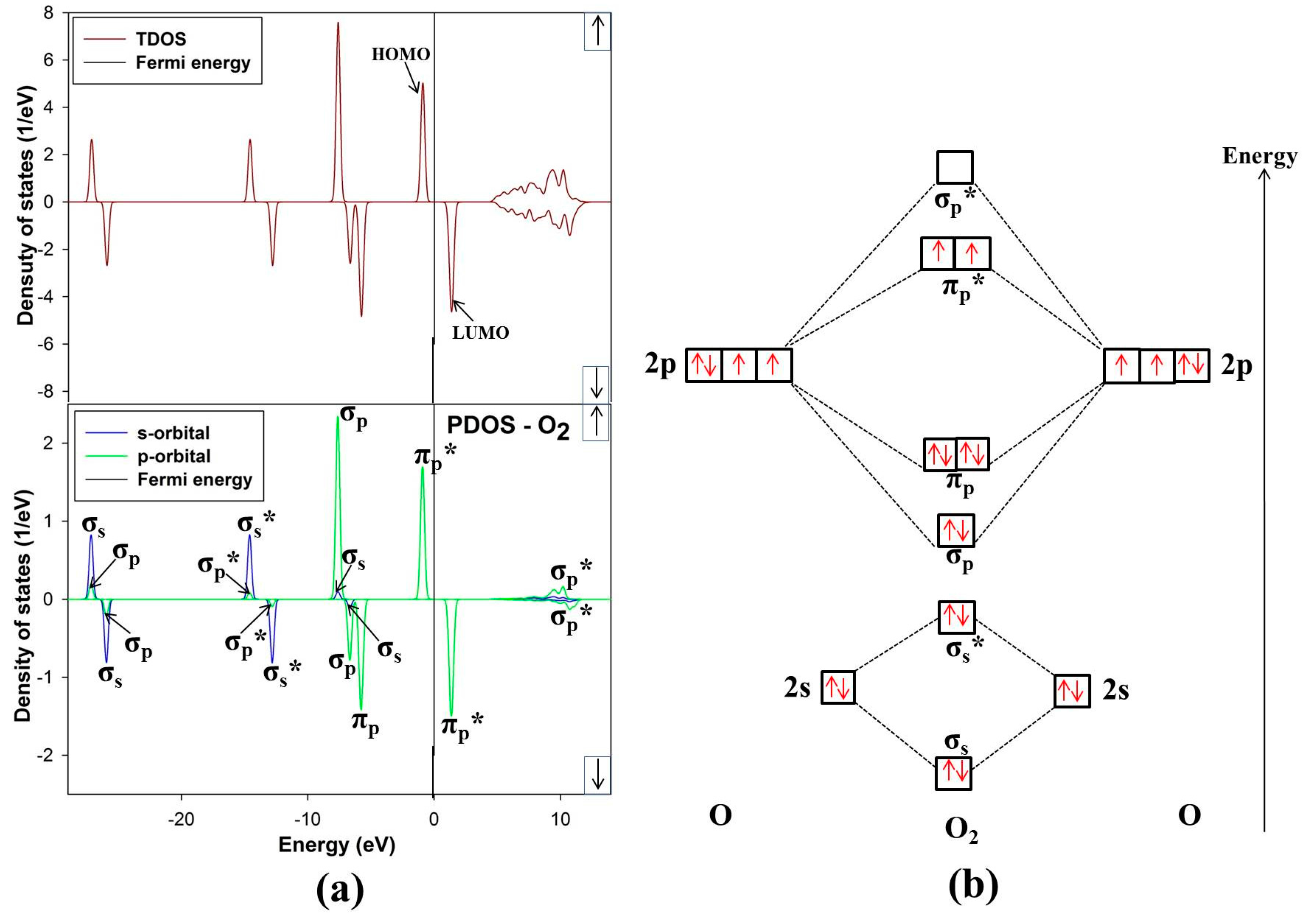
Minerals Free Full Text Ab Initio Studies Of O2 Adsorption On 110 Nickel Rich Pentlandite Fe4ni5s8 Mineral Surface Html


















0 Response to "38 construct the orbital diagram for ni."
Post a Comment