38 what is the basis for exceptions to the aufbau diagram
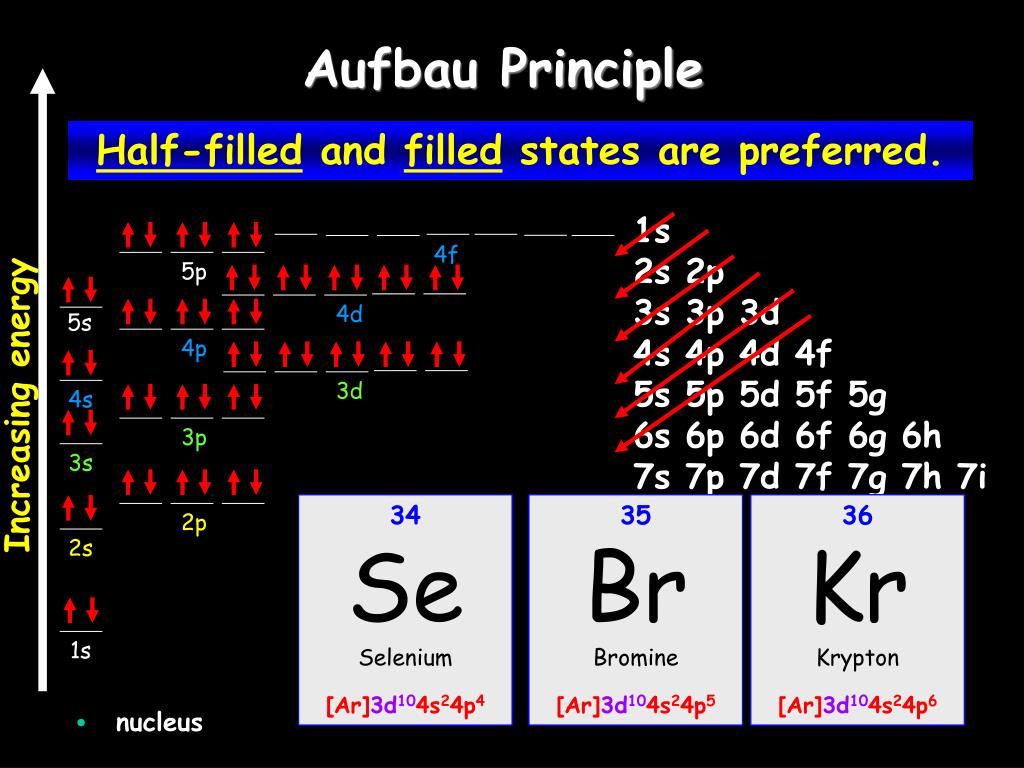
The exception to the Aufbau principle stems from the fact that some atoms prefer to have their higher level energy shells filled over their lower. Exceptions to the Aufbau principle are based on the fact that a few atoms are more stable when their electrons fill or half-fill an electron shell or subshell.According to the Aufbau principle, these electrons should always fill shells and subshells according to increasing energy levels.
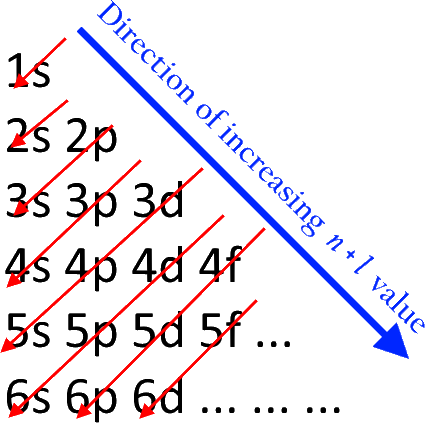
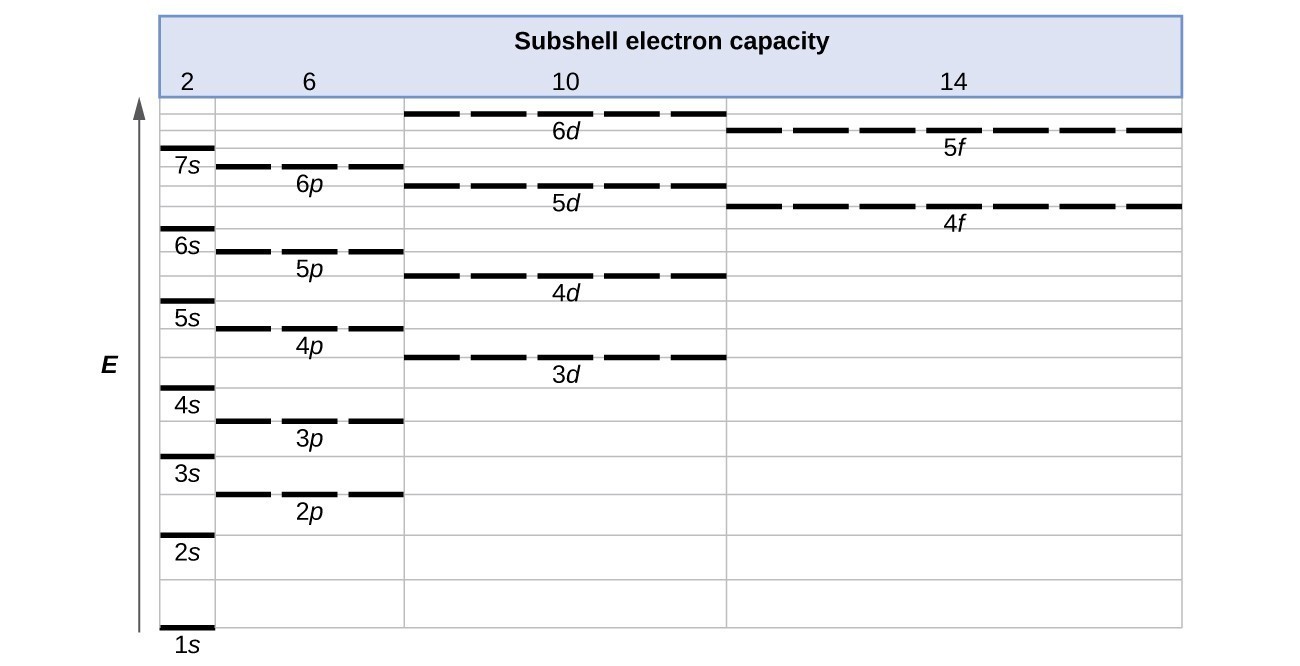
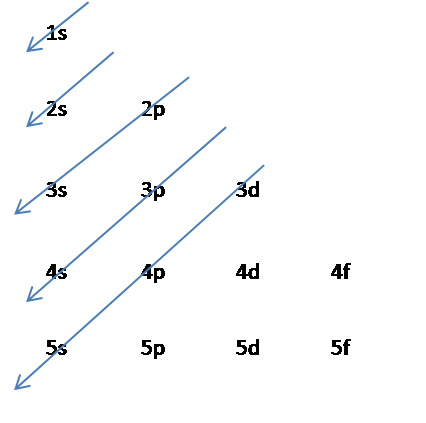
The Aufbau Principle: the (n + l) Rule. We’ve all seen and use the so-called Aufbau Diagram (Figure 1). It is a mnemonic used to remember the order of “filling” of atomic orbitals during the construction of the ground state electron configurations of the elements. The presentation of this diagram is largely disconnected from any physical ...

What is the basis for exceptions to the aufbau diagram
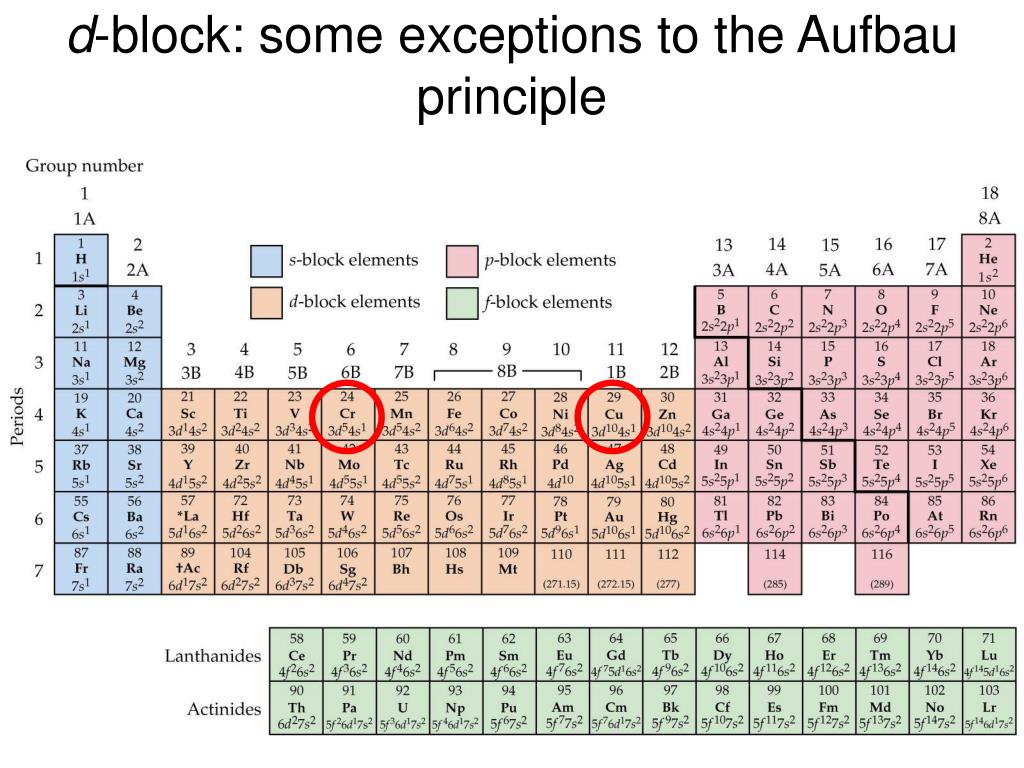
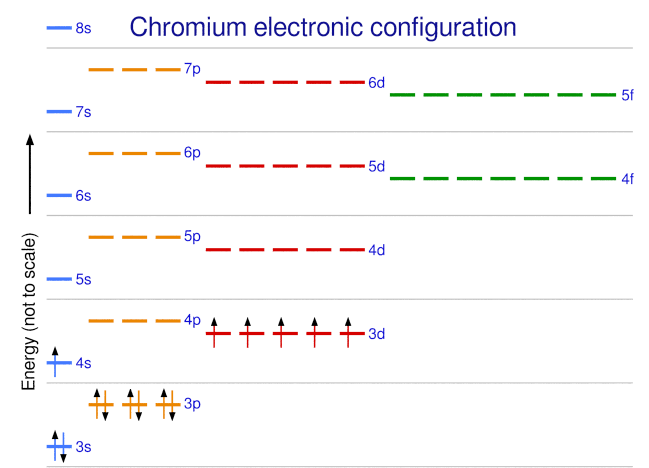
What is the basis for exceptions to the Aufbau diagram?Get the worksheet from here: https://madecalculators.blogspot.com/2020/11/chemistry-more-questions.html What is the basis for exceptions to the aufbau diagram? A. filled and half-filled energy sublevels are more stable than partially-filled energy sublevels B. electron configurations are only probable C. electron spins are more important than energy levels in determining electron configuration D. some elements have unusual atomic orbitals Aufbau Principle Exceptions . Like most rules, there are exceptions. Half-filled and completely filled d and f subshells add stability to atoms, so the d and f block elements don't always follow the principle. For example, the predicted Aufbau configuration for Cr is 4s 2 3d 4, but the observed configuration is actually 4s 1 3d 5. This actually ...
What is the basis for exceptions to the aufbau diagram. 18 Feb 2020 — The aufbau principle states: In the ground state of an atom, atomic orbitals are filled by electrons in the order of their increasing ... A diagram illustrating the order in which atomic orbitals are filled is provided below. Here, ‘n’ refers to the principal quantum number and ‘l’ is the azimuthal quantum number. The Aufbau principle can be used to understand the location of electrons in an atom and their corresponding energy levels. 29 Aug 2021 — Few atoms are more stable when their electrons fill or half-fill an shell or subshell. Where Afbau's only clinched at full filled shells or ...2 answers · 1 vote: Answer:Few atoms are more stable when their electrons fill or half-fill an shell or subshell. ...
4 Jul 2014 · 1 answerSome transition metals don't follow the aufbau, because half-filled orbitals and completely filled orbitals are associated with the stability of ... Most of the exceptions to the electron configuration predicted from the aufbau diagram shown earlier therefore occur among elements with atomic numbers larger than 40. Although it is tempting to focus attention on the handful of elements that have electron configurations that differ from those predicted with the aufbau diagram, the amazing ... aufbau principle. tendency of electrons to enter orbitals of lowest energy first. electron configuration. ... What is the basis for exceptions to the aufbau diagram? Filled and half-filled energy sublevels are more stable than partially-filled energy sublevels. What is the basis for exceptions to the aufbau diagram? some elements have unusual atomic orbitals. People also asked. Which of these powers does the Constitution deny the federal government?
30 May 2018 · 2 answersWith every electron stationed in its own orbital or paired off with each other in the higher energy level, the energy level is balanced and ... What is the basis for exceptions to the Aufbau diagram? Exceptions to the Aufbau principle are based on the fact that a few atoms are more stable when their electrons fill or half-fill an electron shell or subshell. According to the Aufbau principle, these electrons should always fill shells and subshells according to increasing energy levels. Aufbau Principle Exceptions . Like most rules, there are exceptions. Half-filled and completely filled d and f subshells add stability to atoms, so the d and f block elements don't always follow the principle. For example, the predicted Aufbau configuration for Cr is 4s 2 3d 4, but the observed configuration is actually 4s 1 3d 5. This actually ... What is the basis for exceptions to the aufbau diagram? A. filled and half-filled energy sublevels are more stable than partially-filled energy sublevels B. electron configurations are only probable C. electron spins are more important than energy levels in determining electron configuration D. some elements have unusual atomic orbitals
What is the basis for exceptions to the Aufbau diagram?Get the worksheet from here: https://madecalculators.blogspot.com/2020/11/chemistry-more-questions.html
Ppt Quantum Numbers And Orbital Energies Each Atom S Electron Has A Unique Set Of Quantum Numbers To Define It N L Powerpoint Presentation Id 292706




















0 Response to "38 what is the basis for exceptions to the aufbau diagram"
Post a Comment