40 atomic orbital diagram for nitrogen
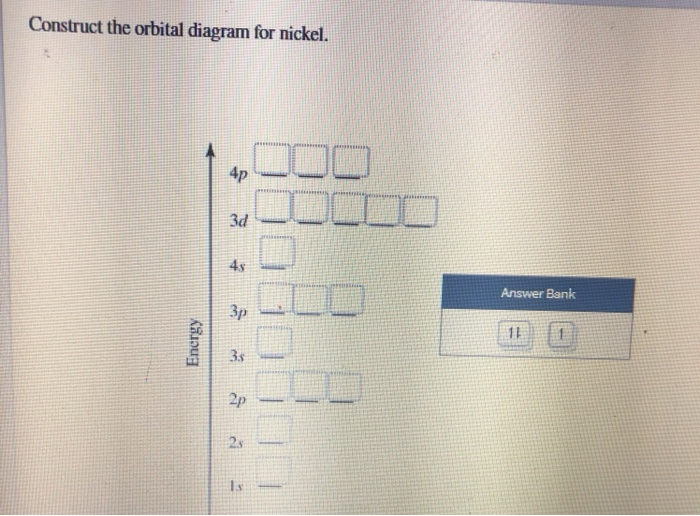
21 Jan 2021 — When we write the electron configuration of N the first two electrons go in the 1s orbital. As 1s can only hold 2 electrons and the other next ... Write the electron configuration of NO molecule in the ground electronic state based on this energy diagram. 6o" Sx 20" 21 Y atom orbitals lo NO molecular ...1 answer · 0 votes: Nitrogen rightarrow 7 rightarrow 1 s^2 2 s^2 2p^3 orbital diagram (2) Ni rightarrow 28 rightarrow 1 s^2 2s^2 2p^6 3s^2 3p^6 3d^8 4s^2 orbital diagram:
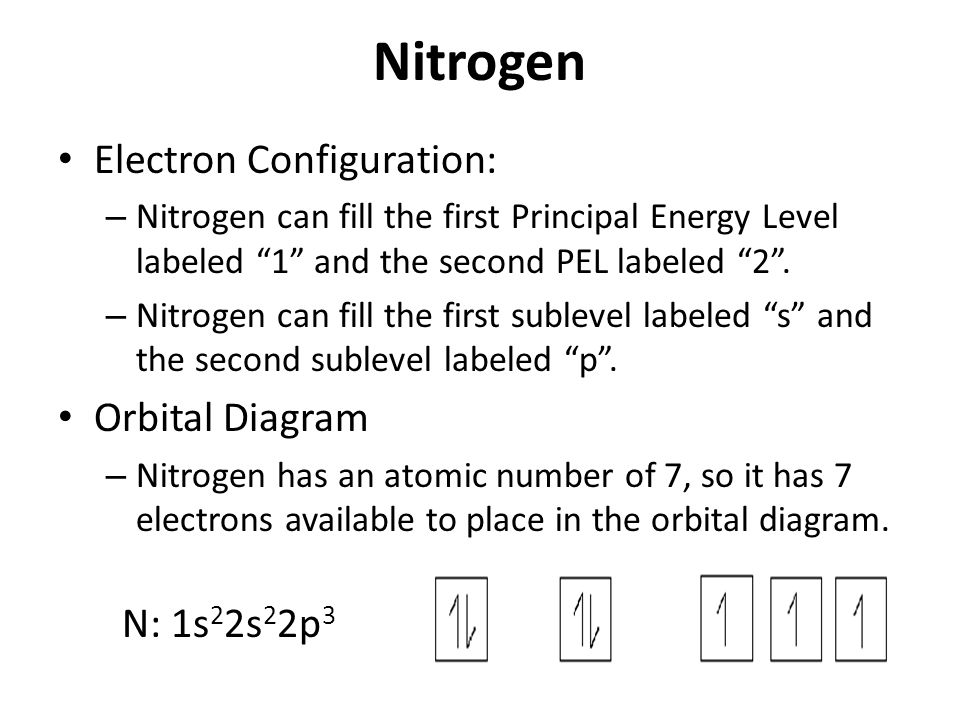
In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons ...18 Nov 2013 · Uploaded by Wayne Breslyn
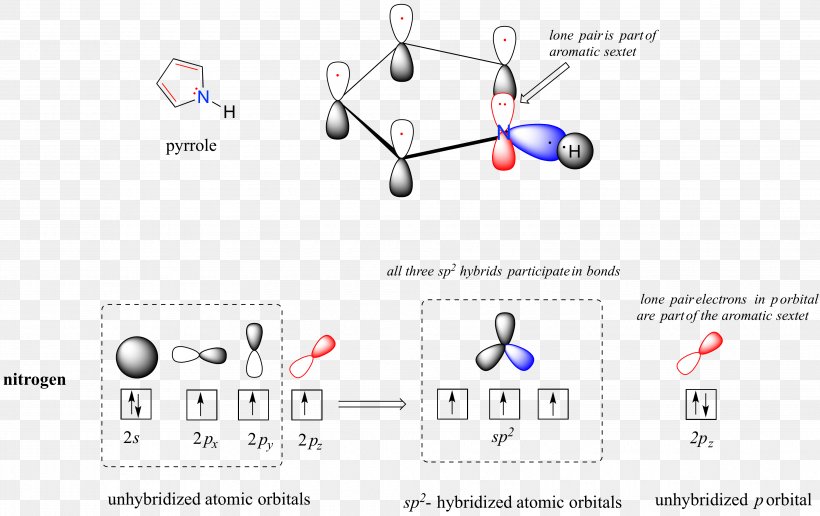
Atomic orbital diagram for nitrogen
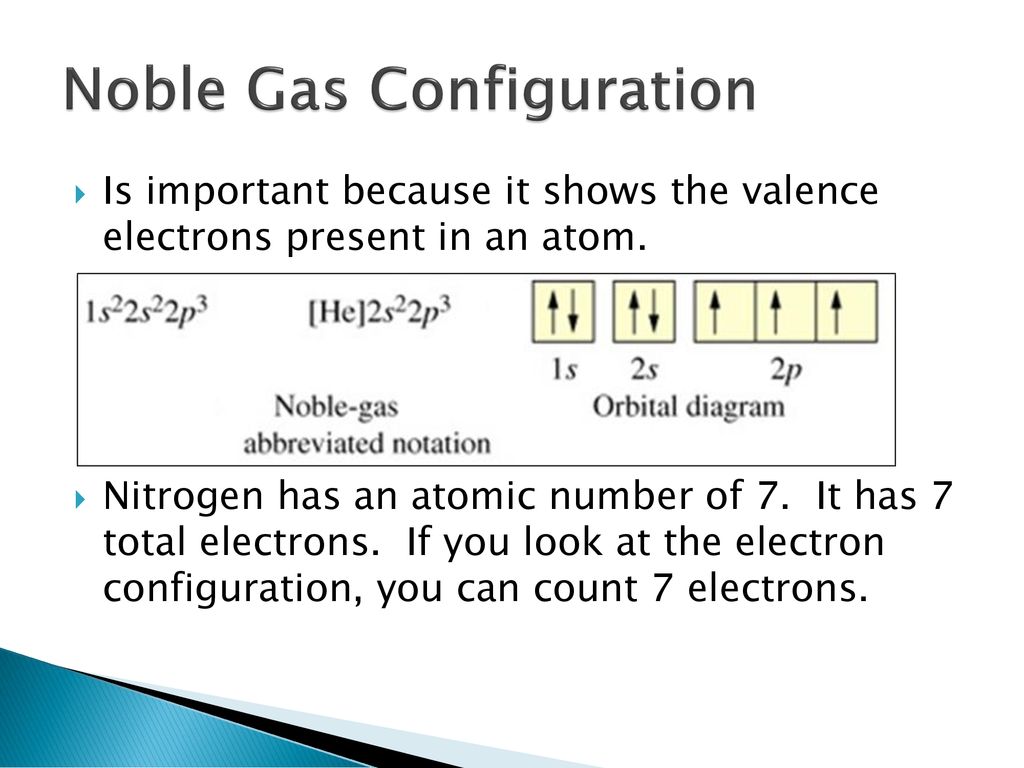
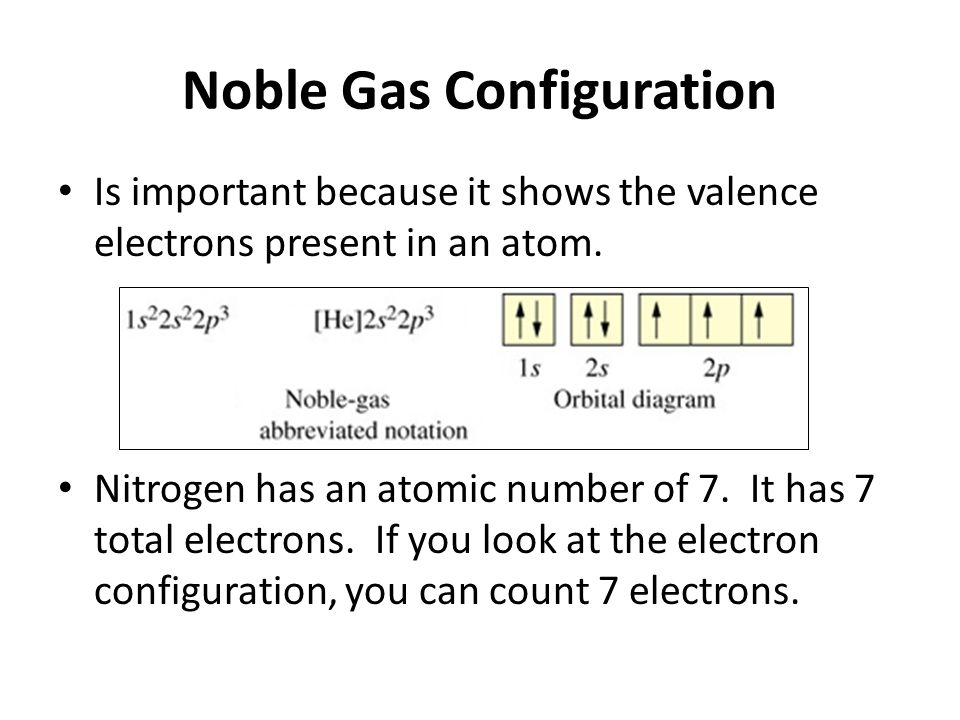
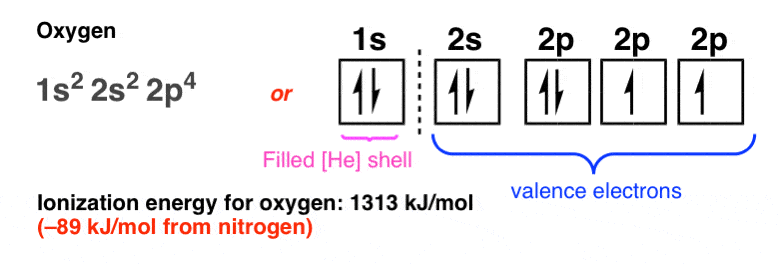
Nitrogen electron configuration is 1s2 2s2 2p3. The two electrons of nitrogen will be in the first orbit and other five electrons will be in the second ...5 Jul 2021 · Uploaded by Wayne Breslyn Create the atomic orbital diagram for nitrogen. Start by adding the appropriate subshells. For example, boron is in the 2p block of the periodic table, and so ... Atomic nitrogen has 5 valence electrons and 4 valence orbitals (2s, 2px, 2py, and 2pz). In the Lewis structure there is a triple bond between the nitrogen ...
Atomic orbital diagram for nitrogen. Atomic Orbital Diagrams: These are also known as electron-in-a-box diagrams. This is a simplified diagram of how the electrons are arranged within the orbitals ...1 answer · Top answer: Nitrogen From the periodic table, we can find Nitrogen which has an atomic number of 7. According to the Aufbau priniciple we have to completely... There are 5 valence electrons in Nitrogen Electron Configuration and it lies at the top of group 15 in the periodic table. Apart from that one ...15 Feb 2021 · Uploaded by Wayne Breslyn Atomic nitrogen has 5 valence electrons and 4 valence orbitals (2s, 2px, 2py, and 2pz). In the Lewis structure there is a triple bond between the nitrogen ... Create the atomic orbital diagram for nitrogen. Start by adding the appropriate subshells. For example, boron is in the 2p block of the periodic table, and so ...
Nitrogen electron configuration is 1s2 2s2 2p3. The two electrons of nitrogen will be in the first orbit and other five electrons will be in the second ...5 Jul 2021 · Uploaded by Wayne Breslyn

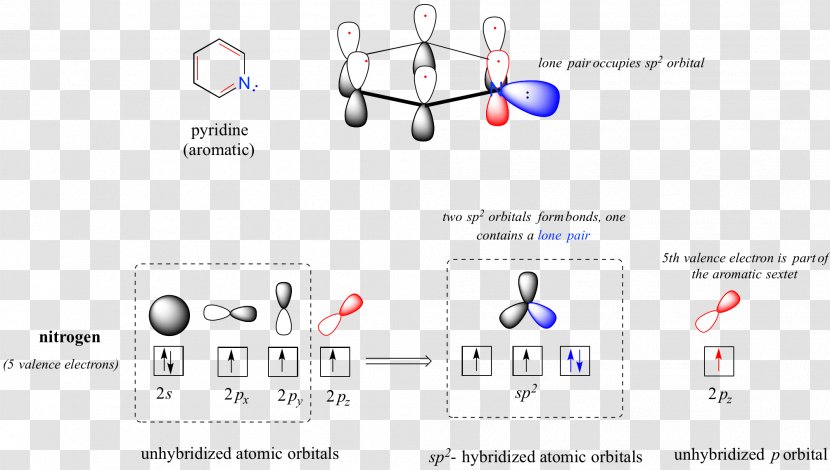
Lewis Structure Atomic Orbital Molecular Orbital Diagram Orbital Hybridisation Png 3870x2439px Lewis Structure Area Aromaticity Atom

Atomic Orbital Electron Configuration Molecular Orbital Diagram Iron Ferric Iron Angle White Electronics Png Pngwing

Above Orbital Diagram Shows The Electron Configuration Of Nitrogen Atom Which Rule Does Not Support This

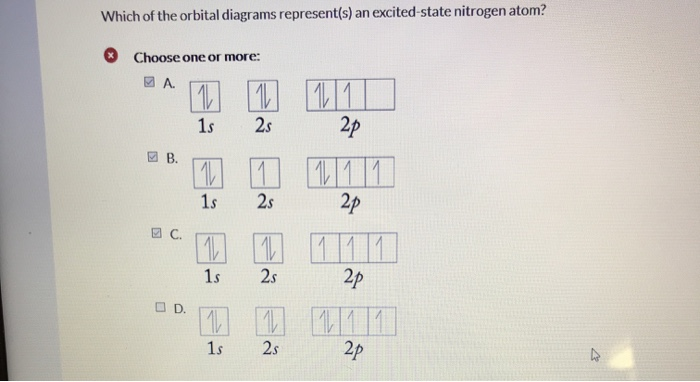
Which Of The Orbital Diagrams Represent S An Excited State Nitrogen Atom Choose One Or More 1 2p Homeworklib

Molecular Orbital Diagram White Atomic Orbital Molecular Orbital Theory Molecule Bond Order Electron Valence Electron Nitrogen Molecular Orbital Diagram Diagram Molecular Orbital Png Pngwing

The Ground State Valence Shell Electrons Configuration Of Nitrogen Atom Can Be Represnted As Youtube

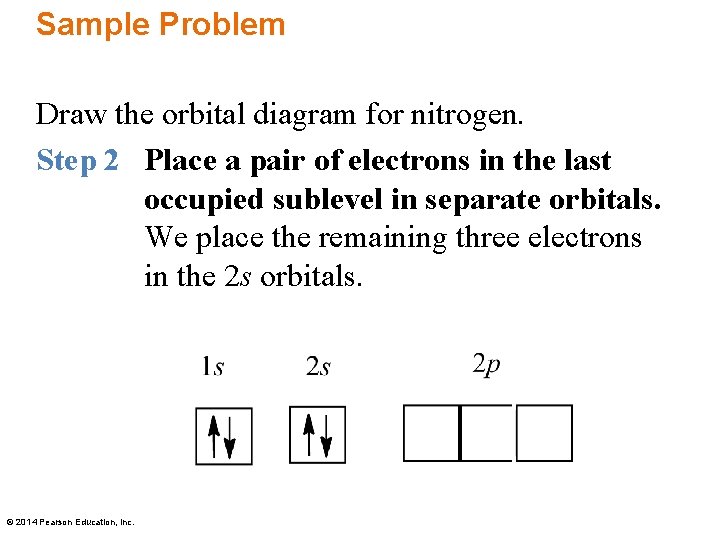
Science Starter Drawn An Orbital Diagram And Give The Electron Configuration For N Nitrogen Ppt Download















0 Response to "40 atomic orbital diagram for nitrogen"
Post a Comment