40 lewis dot diagram for copper
A step-by-step explanation of how to draw the Cu(OH)2 Lewis Dot Structure.For Cu(OH)2 we have an ionic compound and we need to take that into account when we... You can't simply draw a Lewis dot structure for a metal, you have to know the metal. In the case of copper, the electron from the "s" shell is moved to fill the "d" shell, so there is only one electron in the "s" shell. One would simply write: Cu·. If you had a pair of coppers, you would write Cu:Cu.
The lewis structure for an element or ion can be drawn by representing the electrons of the valence shell as dots. The octet of the atom should be kept in mind ...1 answer · Top answer: The atomic number of Cu=29 The electronic configuration of its valence shell =3d10,4s13d10,4s1 Its lewis dot structure can be drawn as...
Lewis dot diagram for copper
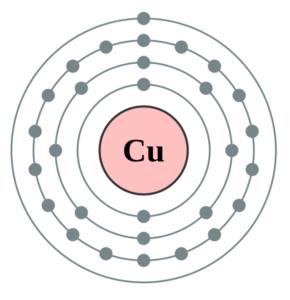
The Lewis formalism used for the H 2 molecule is H:H or H—H. The former, known as a 'Lewis dot diagram,' indicates a pair of shared electrons between the atomic symbols, while the latter, known as a 'Lewis structure,' uses a dash to indicate the pair of shared electrons that form a covalent bond. Answer: lewis dot diagram for cuo is a bulletin dot above cu. Explanation: Molecular Formula : CuO. name : Copper Oxide or cupric oxide Bohr Model of Copper
Lewis dot diagram for copper. A lewis electron dot diagram or electron dot diagram or a lewis diagram or a lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The lewis dot diagramstructure for copper would be cu with a dot on top because if you look at the shell model of copper there is only one dot on ... Answer: Copper (II) sulfate is CuSO4 The structure is tricky, because CuSO4 is not covalent, it is ionic. So it would be: There wouldn't be a lewis structure for the whole thing. A. Write the electron configuration and orbital diagrams of Be2+, Al3+, Ca.B. What is a Lewis dot symbol ? To what elements does the symbol mainly apply?for ...1 answer · 0 votes: Chemical symbol: Cu Number of electrons: 29 1 on the valence shell TTUchme1010 teaches viewers how to draw the lewis dot structure for sulfate. The formula for this is SO4^2-. 2- means we will have to add 2 electrons into the lewis dot structure. First, we will have Sulfur in the middle with Oxygen surrounding it. Sulfur is in group 6A so it have 6 valence electrons and oxygen has six, so fill this all in around the elements.
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. Copper is a metal that occurs naturally throughout the environment, in rocks, soil, water, and air. Copper is an essential element in plants and animals (including humans), which means it is necessary for us to live. Therefore, plants and animals must absorb some copper from eating, drinking, and breathing. Copper is used to make many different kinds of products like wire, plumbing pipes, and ... Jun 5, 2019 — Each Lewis dot symbol consists of the chemical symbol for an element surrounded by dots that represent its valence electrons. In the formula for copper (II) phosphate, the number of phosphorus atoms is. two. ... Group 7A. How many dots will appear in the Lewis dot structure for an element from Group 1A of the periodic table? one. Chalk is primarily composed of calcium Carbonate. The formula of calcium carbonate is. CaCO3.
What is the Lewis dot structure for copper? Lewis Structure: The lewis structure for an element or ion can be drawn by representing the electrons of the valence shell as dots. The octet of the atom should be kept in mind while drawing the lewis structure of any element. The copper ion exist in two possible oxidation state and these are Cu+ and ... Lewis dot diagram for hcn. What is the lewis structure of hcn. How to draw hcn lewis structure. The lewis dot diagramstructure for copper would be cu with a dot on top because if you look at the shell model of copper there is only one dot on the outer ring. In the formal way we find how many electrons we have step 1 how many each atom needs ... Answer: Copper wants to donate 2 electrons for a full valence shell (see it's column in the periodic table). Lewis Dot Structure (the 8 statements below preceded by dashes AND the Lewis dot periodic table were copied from Bonds and the Lewis Dot Structure and are not original to me!!) -All eleme... A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ...

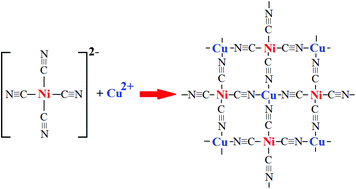
Chemistry And Structure By Design Ordered Cuni Cn 4 Sheets With Copper Ii In A Square Planar Environment Dalton Transactions Rsc Publishing
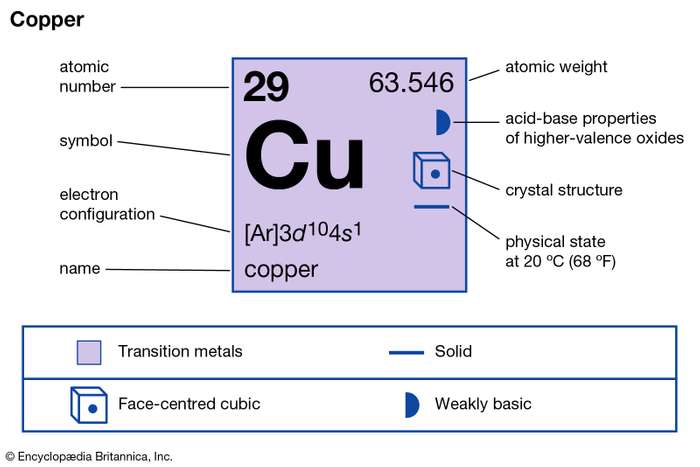
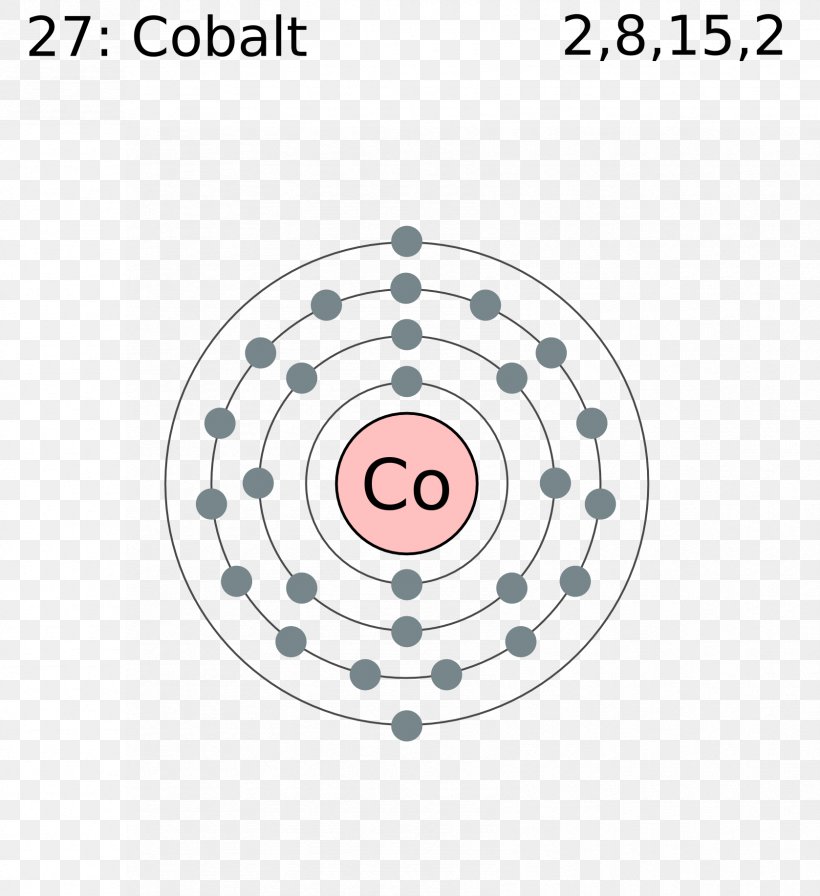
NOTE: Copper is an exception to the rules for writing electron configurations! Video: Cu, Cu +, and Cu 2+ Electron Configuration Notation In writing the electron configuration for Copper the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Copper go in the 2s orbital.
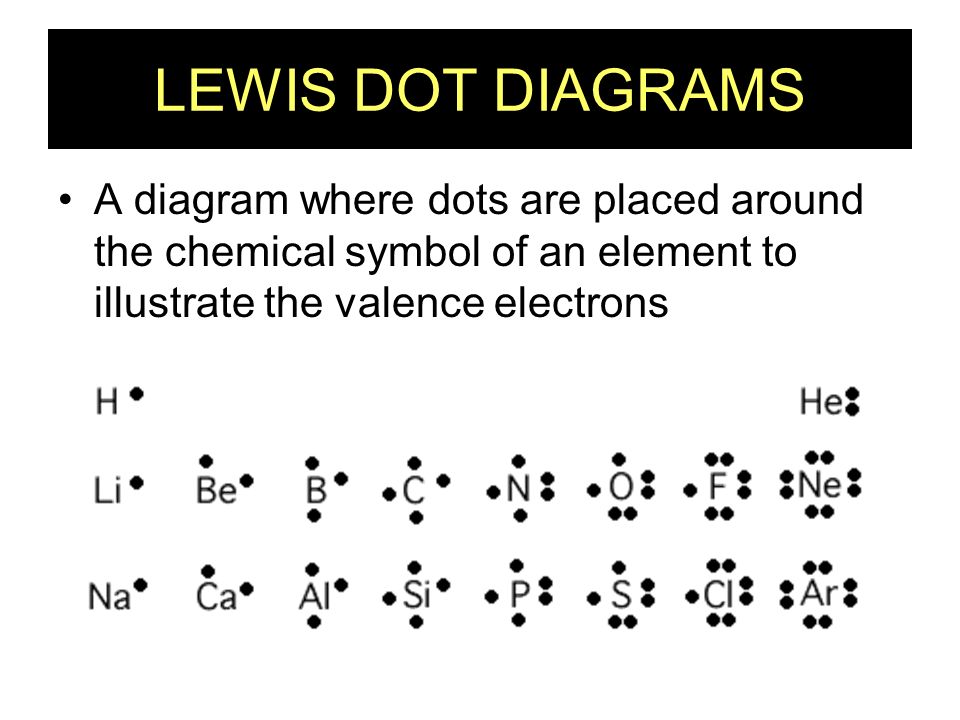
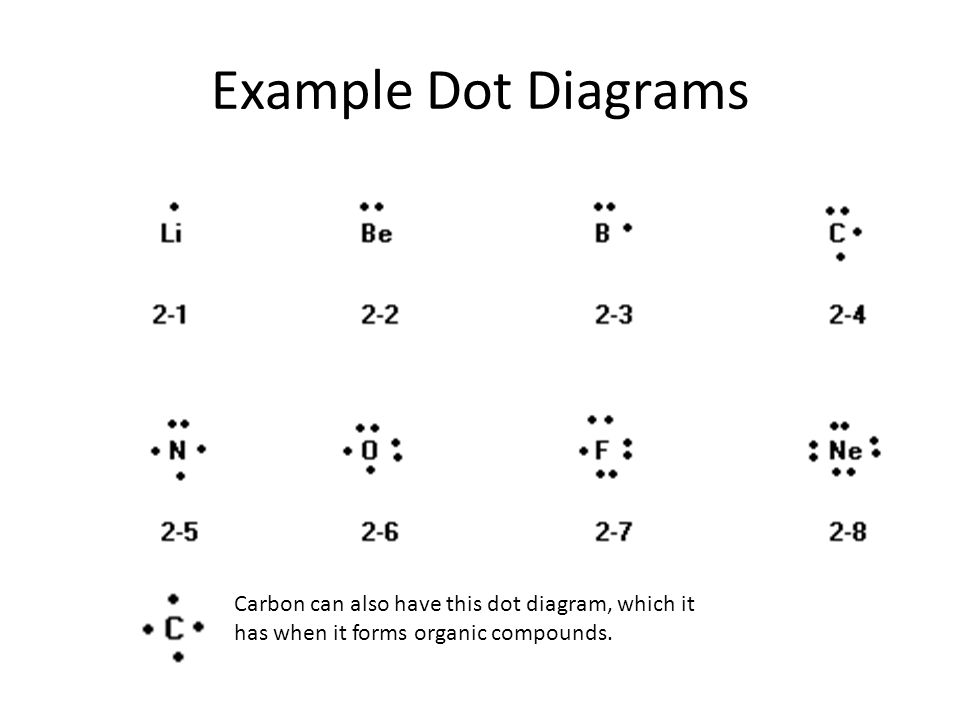
Lewis-Dot Diagrams •Dots are placed one at a time on the four sides of the symbol, then paired until all valence electrons are used… -Maximum of 8 electrons will be around the symbol •d sublevel electrons are not valence electrons -they are in a lower energy level! 5.3 Electron Configuration
Cupric chloride, for injection, is a sterile, nonpyrogenic solution intended for use as an additive to solutions for Total Parenteral Nutrition (TPN). Copper chloride appears as a yellowish-brown powder (the anhydrous form) or a green crystalline solid (the dihydrate). Noncombustible but hydrogen chloride gas may form when heated in a fire.
Electron Dot Diagrams There is another model called the electron dot or Lewis diagram. This system represents an atom and its valence electrons. The electron dot diagram uses the symbol of the element to replace the nucleus and inner shell electrons. The electrons in the valence shell are shown as dots placed around the symbol.
What is the lewis dot structure of C2H5F? Chemistry. A mixture of copper sulfate and water is heated, leaving a residue of copper sulfate in the container. ... sketch the atomic structure of copper and discuss why it is a good conductor and how its structure different from Ge and Si
Best Answer. Copy. The Lewis dot diagram (structure) for copper would be Cu with a dot on top, because if you look at the shell model of copper, there is only one dot on the outer ring. So the ...
katie - 10/22/2006 4:13:22 am. help!! i need a drawing of a lewis dot diagram and of nucleus and electrons in their proper energy levels! reply
What is the Lewis dot diagram for copper? Organic Chemistry Lewis Structures and Bonding Lewis Dot Diagram. 1 Answer Karen L. Aug 2, 2016 Chemical symbol: Cu Number of electrons: 29 1 on the valence shell. Answer link. Related questions. How is the Lewis structure of an ion written? ...
The Lewis Structure, or Lewis Dot Diagram, shows the bonding between atoms of a molecule and any electrons that may exist. The Lewis Structure for Li is Li with one dot to the right of the element.
May 8, 2017 · 1 answerCopper wants to donate 2 electrons for a full valence shell (see it's column in the periodic table). · Sulfur wants to accept 2 electrons for a full valence ...How to determine the Lewis dot structure of copper(II ...1 answerDec 19, 2017What is the Lewis dot structure of CuSO4? - Quora4 answersAug 26, 2015More results from www.quora.com
LEWIS DOT DIAGRAMS Name Lewis diagrams are a way to Indicate the number of valence electrons aroun an atom. A\5Ö are all examples of this type of diagram. Draw Lewis dot diagrams of the following . l. calcium 2. potassiürn rgon 4. aluminum 2-3 -B 5, bromine 6. carbon . 7. helium 8. oxygen 9. phosphorus . hy rogen
7.Copper has two naturally occurring isotopes. Information about the two isotopes is shown in the table below. In the space in your answer booklet, show a numerical setup for calculating the atomic mass of copper. 8.In the box below, draw a Lewis electron-dot diagram for an atom of boron.
The arrangement of the valence electrons for oxygen and hydrogen when they bond show that the Lewis dot structure represents. ... Copper. Which of these describes the electron behavior necessary for sodium to bond with another element? Sodium will lose 1 electron and form an ion. The formation of an ionic bond involves the.

What S In A Name When Two People Use Different Names For The Same Thing Misunderstood Words Are Apt To Happen The British And Americans Often Get Confused Ppt Download
Jan 25, 2021 — You can understand the valence electrons with better insight by the dot diagram. We call the dot diagram as Lewis dot diagram for valence ...
Bohr Model of Copper
Answer: lewis dot diagram for cuo is a bulletin dot above cu. Explanation: Molecular Formula : CuO. name : Copper Oxide or cupric oxide
The Lewis formalism used for the H 2 molecule is H:H or H—H. The former, known as a 'Lewis dot diagram,' indicates a pair of shared electrons between the atomic symbols, while the latter, known as a 'Lewis structure,' uses a dash to indicate the pair of shared electrons that form a covalent bond.

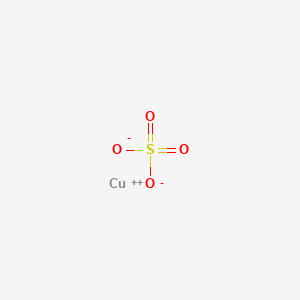
Copper Ii Sulfide 99 8 Metals Basis Alfa Aesar 50g Copper Ii Sulfide 99 8 Metals Basis Alfa Aesar Fisher Scientific
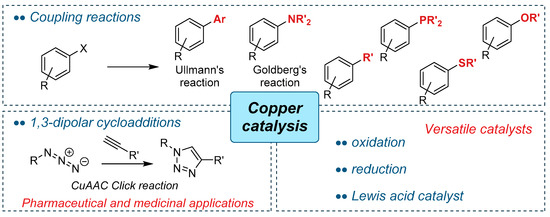
Catalysts Free Full Text Bio Based Catalysts From Biomass Issued After Decontamination Of Effluents Rich In Copper An Innovative Approach Towards Greener Copper Based Catalysis
















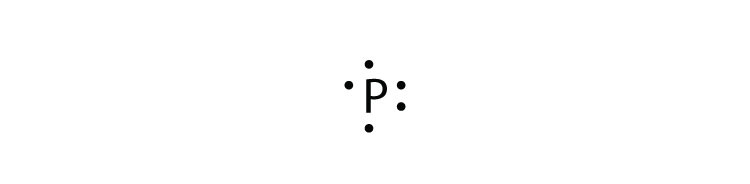

0 Response to "40 lewis dot diagram for copper"
Post a Comment