41 ethylene molecular orbital diagram
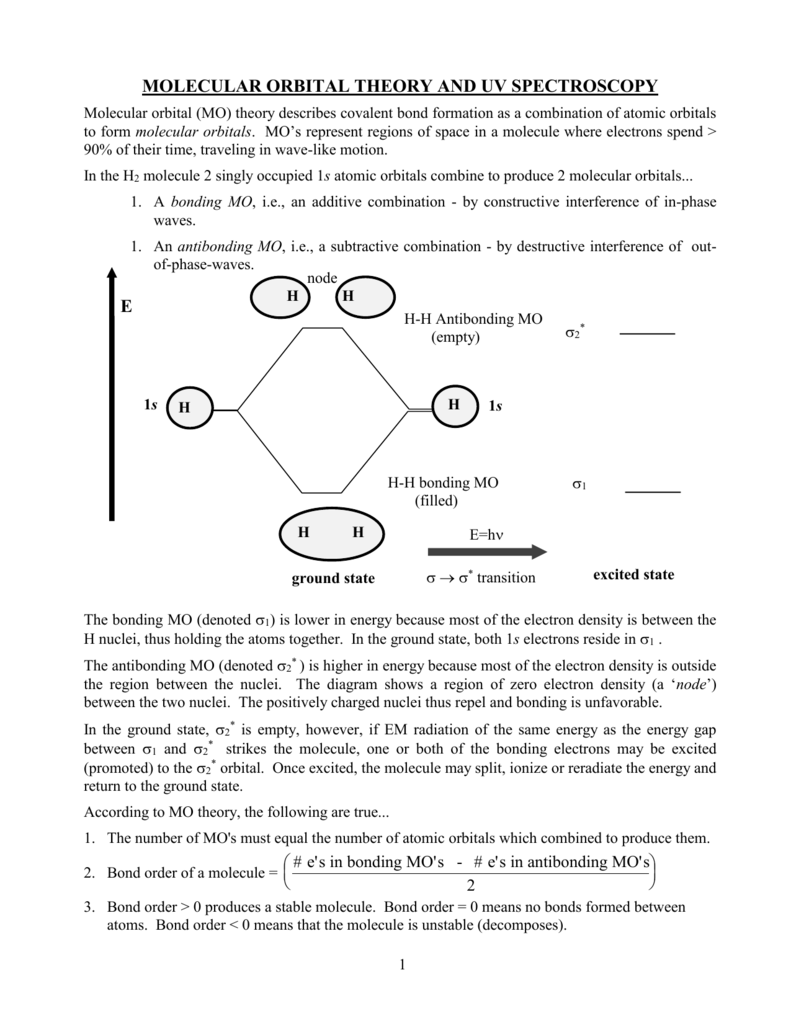
Molecular Orbital (MO) Theory (continued 1) • Filling of MOs with electrons is governed by the same rules as for atomic orbitals • Aufbau principle - Fill MOs beginning with the lowest energy unoccupied molecular orbital • Pauli exclusion principle - No more than two electrons can be accommodated in a MO, and their spins must be paired 19:16In this video we will generate a qualitative MO diagram of ethene through a fragment molecular orbital ...31 Mar 2020 · Uploaded by Some Chemistry Lecture Videos I Made
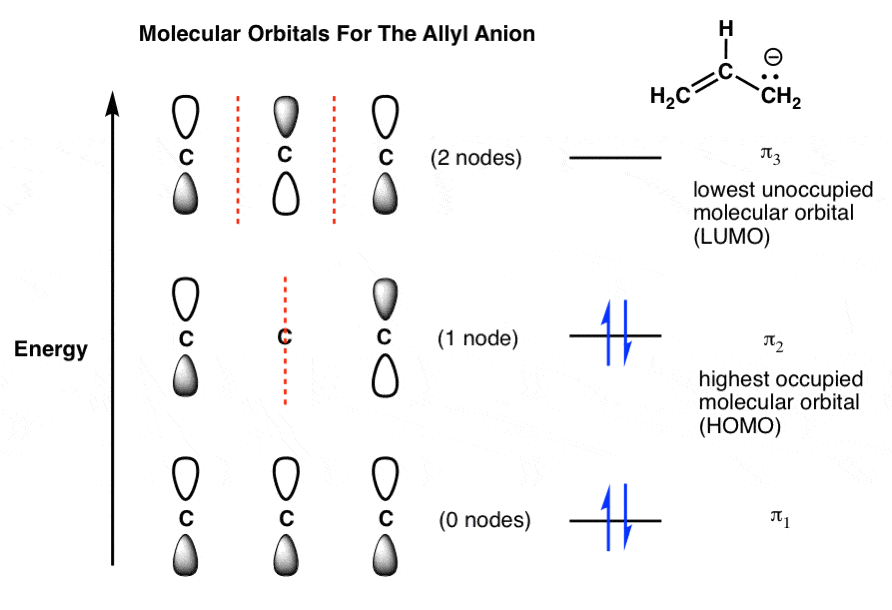
Pi Molecular Orbitals of 1,3,5-Hexatriene. With a single sigma bond separating the pi bonds of 1,3,5-hexatriene it is a conjugated system and some of the pi electron density will be delocalized between each of the C-C bonds, not just those written as double bonds in the Lewis structure. There are six adjacent carbon atoms involved in the pi ...
Ethylene molecular orbital diagram
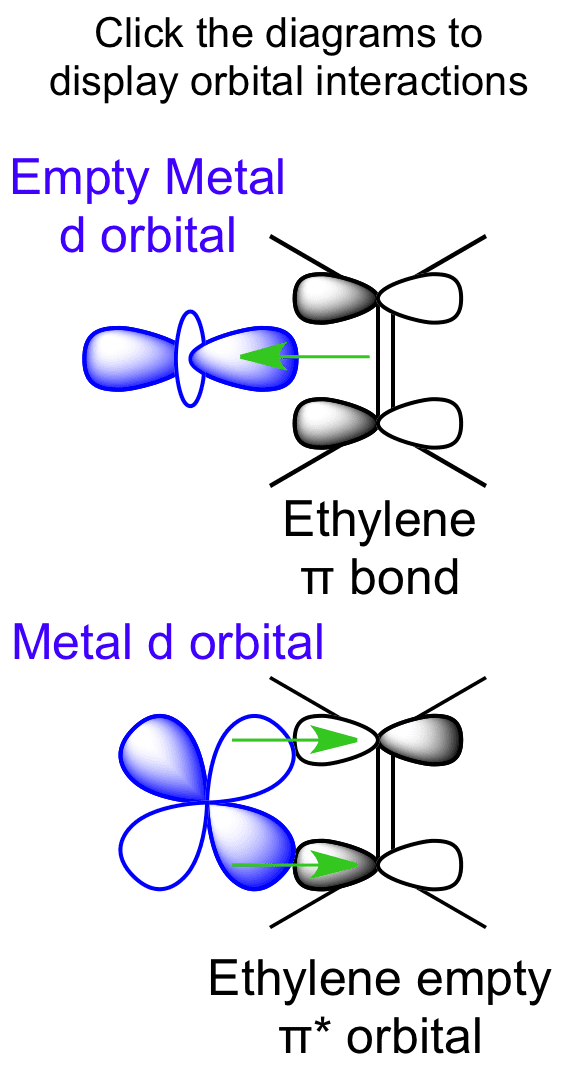
3:26This video shows that the pi framework and sigma framework of ethylene are distinct orbital sets.18 Jan 2010 · Uploaded by jeffrey Moore Interactions between Ethylene Molecular Orbitals and Metal d Orbitals. could not be loaded. Script error: Forbidden is not defined. Ethylene is capable of acting as a ligand as the C=C π bond can donate electron density to an empty metal d orbital, forming a σ bond. A filled metal d orbital is capable of donating electron density into the C=C ... In ethylene, each carbon combines with three other atoms rather than four. There is a formation of a sigma bond and a pi bond between two carbon atoms. C2H4 Molecular Geometry And Bond Angles. C2H4 molecular geometry is said to be planar in structure while the sp 2 orbitals are placed at a bond angle of 120 o.
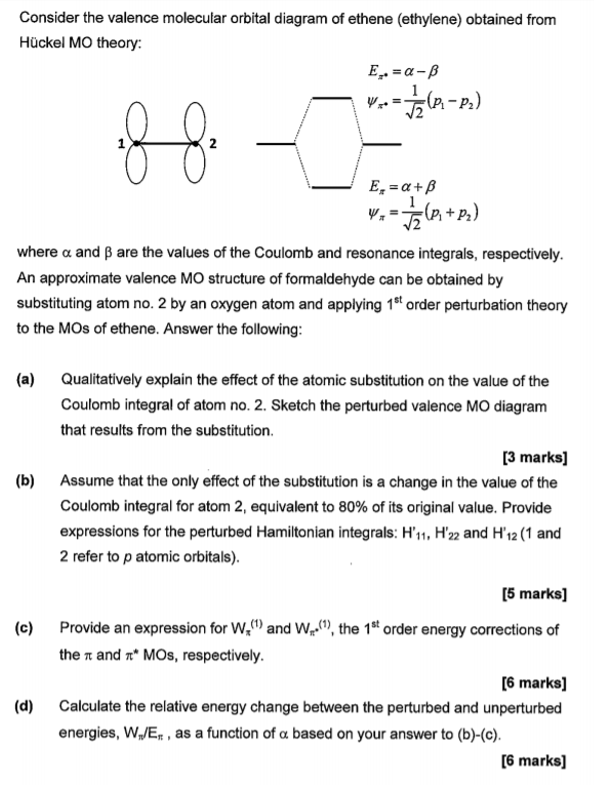
Ethylene molecular orbital diagram. A molecular orbital diagram of ethene is created by combining the twelve atomic orbitals associated with four hydrogen atoms and two sp 2 hybridized carbons to give twelve molecular orbitals. Six of these molecular orbitals (five sigma & one pi-orbital) are bonding, and are occupied by the twelve available valence shell electrons. 5:11Sigma pi bond formation Orbital overlap concept ncert. ... Q. Draw molecular orbital diagram for ethane, ethene ...5 Jun 2020 · Uploaded by Chemistry Explorers- Anshudeep tomar The correct Lewis structure for ethene is shown below: In the molecule ethene, both carbon atoms will be sp2 hybridized and have one unpaired electron in a non-hybridized p orbital. These p-orbitals will undergo parallel overlap and form one σ σ bond with bean-shaped probability areas above and below the plane of the six atoms. Molecular orbitals for ethene (ethylene) In the bonding pi orbital, the two shaded lobes of the p orbitals interact constructively with each other, as do the two unshaded lobes (remember, the arbitrary shading choice represents mathematical (+) and (-) signs for the mathematical wavefunction describing the orbital). There is increased electron ...
occupied molecular orbitals and N/2 unoccupied ones. For the ground state, we of course occupy the lowest energy orbitals. ... Hückel theory for ethylene, we find that a single ethylene double bond has an energy EC=C = 2α+ 2β Thus, if benzene simply had three double bonds, we would expect it to have a total energy of Bonding orbitals in Ethylene (Ethene) sp. 2. 1 model in this collection. Use getProperty "modelInfo" or getProperty "auxiliaryInfo" to inspect them. Use "set autoLoadOrientation TRUE" before loading or "restore orientation DEFAULT" after loading to view this orientation. spinFPS is set too fast (30) -- can't keep up! Molecular Orbitals: Example 1: Ethylene E E H2CCH2 p 2 sp2 1s p 2 sp2 1s σ σ* π π* A.O. A.O. C C HHC C C from other end of double bond A.O. means atomic orbitals (s, sp2, p) M.O. means molecular orbitals (σ , π ) C from left end of double bond M.O. Looking at both sigma and pi bonds Using Symmetry: Molecular Orbitals One approach to understanding the electronic structure of molecules is called Molecular Orbital Theory. • MO theory assumes that the valence electrons of the atoms within a molecule become the valence electrons of the entire molecule.
If released to air, a vapor pressure of 5.21X10+4 mm Hg at 25 °C indicates ethylene will exist solely as a gas in the atmosphere. Gas-phase ethylene will be degraded in the atmosphere by reaction with photochemically-produced hydroxyl radicals; the half-life for this reaction in air is estimated to be 2 days. The other sp2 hybrid orbitals form sigma bonds between C and H, therefore, leading to C-H single bonding structure. C2H4 Molecular Orbital (MO) Diagram. The molecular orbital theory is a concept of quantum mechanics where atomic linearly combines to form molecular orbitals and we describe the wave nature of atomic particles. A molecular orbital diagram of ethene is created by combining the twelve atomic orbitals associated with four hydrogen atoms and two sp 2 hybridized carbons to give twelve molecular orbitals. Six of these molecular orbitals (five sigma & one pi-orbital) are bonding, and are occupied by the twelve available valence shell electrons. 3:51In order to use FMO analysis on a molecule, like ethylene or formaldehyde, the first step is to determine the ...30 Jan 2019 · Uploaded by Erland Stevens
Molecular Orbitals: Ethene (Ethylene) Ethylene MOs. Ethylene is the simplest molecule that has a double bond. As we saw from the valence bond model, we should find the presence of a σ-bond framework, and a π-bond between carbons. Jmol._Canvas2D (Jmol) "Ethylene" [x]
Molecular orbitals for ethene (ethylene) In the bonding pi orbital, the two shaded lobes of the p orbitals interact constructively with each other, as do the two unshaded lobes (remember, the arbitrary shading choice represents mathematical (+) and (-) signs for the mathematical wavefunction describing the orbital). There is increased electron ...
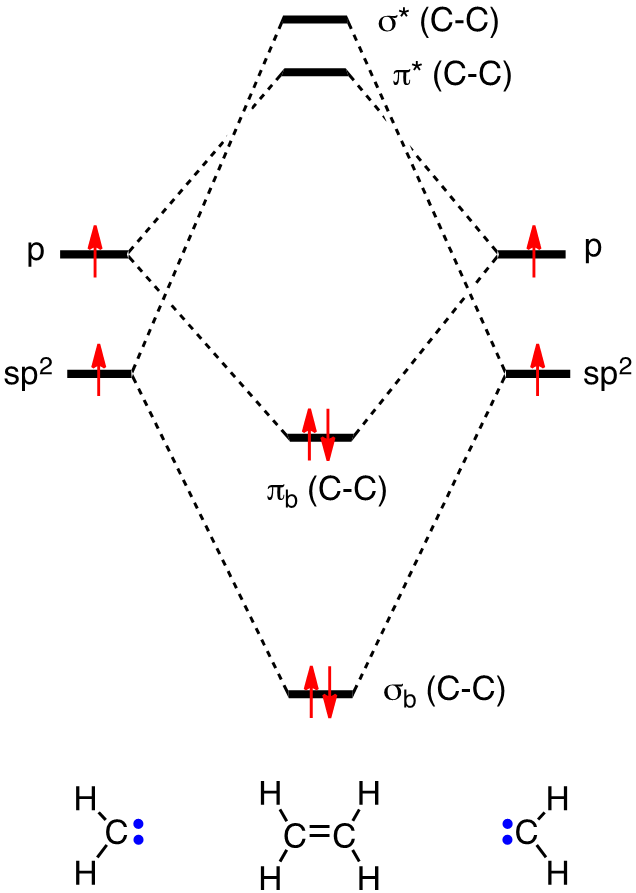
In chapter 1 we saw that the molecular orbitals of H 2 are created by the combination of 1s orbitals. The in-phase combination gave the bonding orbital. The out-of-phase combination the anti-bonding orbital. For ethene, the σ framework is created by the interaction of the sp 2 hybrid orbitals of the C atoms and H1s orbitals.
Ethane. • As molecules get bigger constructing the molecular orbitals. becomes more challenging. • Insights into bonding of larger molecules can be attained by. combining fragments with well defined MO's... through orbital. mixing. • In this manner, ethane can be constructed from MO's of two. pyramidal CH3 groups.
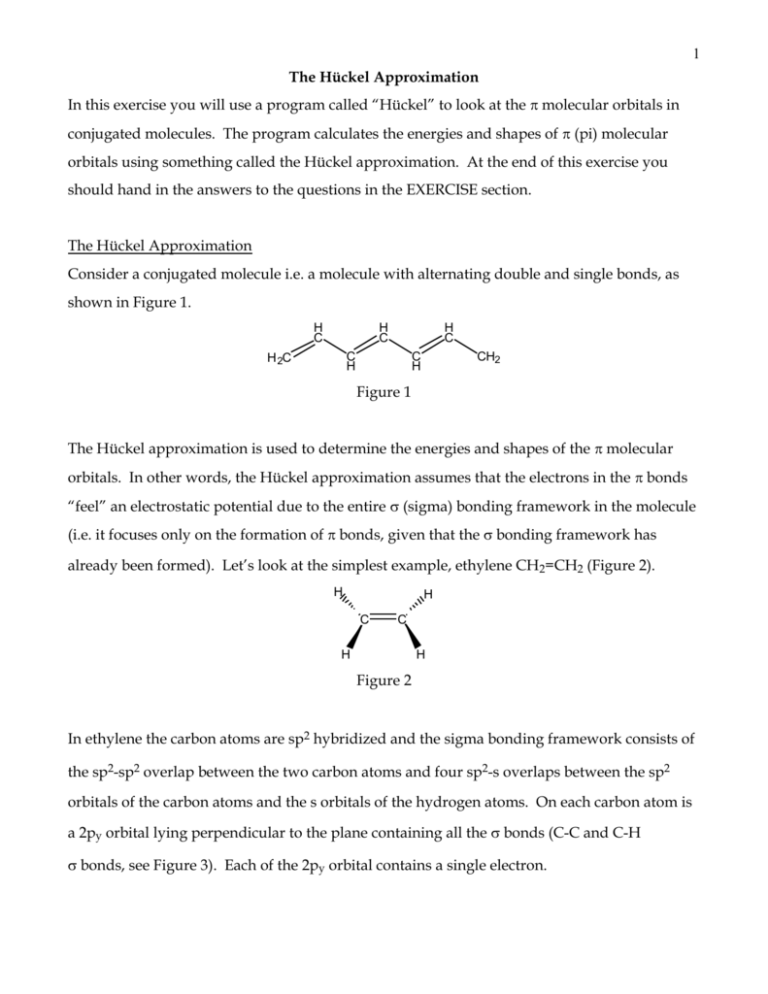
determine the shapes of the π molecular orbitals in ethylene. 6 Open the Hückel program. At the top of the window is a menu with the following options - File, Edit, View, Calculation, Help Below the menu is a tool bar. Below the tool bar is a page with a grid. Tool Bar
Ethene This sideways overlap also creates a molecular orbital, but of a different kind. In this. Ethylene is the simplest molecule that has a double bond. As we saw from the valence bond model, we should find the presence of a σ-bond framework, and a . Bonding orbitals in Ethene (Ethylene) sp2. sp2 hybrids. ethylene orbitals.
The hybrid orbitals are more prominent outward so that their ability to overlap is stronger than that of normal orbitals. Molecular Formula: A chemical formula is a brief way of expressing the number and type of atoms that make up a particular chemical compound.
Chad introduces Pi Molecular Orbitals using Ethylene, drawing Bonding and Antibonding Molecular Orbitals and identifying the HOMO and LUMO.I've created an or...
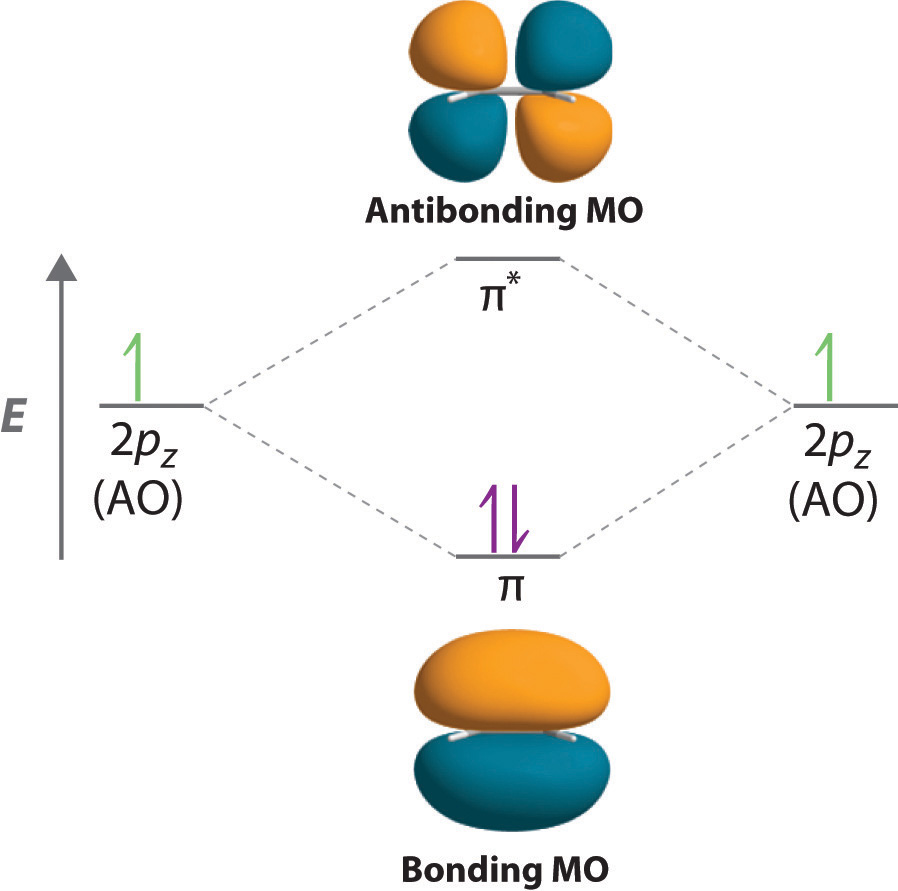
In ethylene there are two adjacent carbon atoms involved in the pi system and the combination of a p orbital from each of these atoms will result in two pi molecular orbitals: ψ 1 and ψ 2 *, (also referred to as π 1 and π 2 *).. ψ 1 is a bonding molecular orbital, is occupied in the ground state, and is the Highest Occupied Molecular Orbital (HOMO). ψ 2 * is an antibonding molecular ...
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula C 2 H 4 or H 2 C=CH 2.It is a colorless flammable gas with a faint "sweet and musky" odor when pure. It is the simplest alkene (a hydrocarbon with carbon-carbon double bonds).. Ethylene is widely used in the chemical industry, and its worldwide production (over 150 million tonnes in 2016) exceeds that of any other organic ...
Molecular Orbitals in Conjugated Systems. According to the frontier orbital theory, the chemistry of conjugated π systems is largely determined by the HOMO and LUMO π orbitals in the reactant molecules.The outcome of reactions involving interaction of π orbitals can be rationalized using the concepts of orbital phase and orbital symmetry.
The molecular orbital diagram for the π-molecular orbitals of butadiene as a result of combining the π-molecular orbitals of two ethene molecules. This shows .Bonding orbitals in Ethene (Ethylene) sp 2 Background: Use the buttons to display the sp 2 orbitals that make up the sigma framework and the remaining p orbitals which form the pi-bond.
Molecular Orbital Diagrams simplified. Megan Lim. Oct 26, 2016 · 3 min read. Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding ...
14 molecular orbitals read in model 2. 0 molecular orbitals read. Mulliken charges found for Model 2. Molecular dipole for model 2 = {0, 0, 0} Time for openFile (./mo/ethylene.log): 101 ms. reading 12 atoms. ModelSet: haveSymmetry:false haveUnitcells:false haveFractionalCoord:false. 2 models in this collection.
**The bonding π orbital is the lower energy orbital and contains both p electrons (with opposite spins) in the ground state of the molecule. The region of greatest probability of finding the electrons in the bonding π orbital is a region generally situated above and below the plane of the σ-bond framework between the two carbon atoms.

Molecular Orbital Diagram White Atomic Orbital Molecular Orbital Theory Molecule Bond Order Electron Valence Electron Nitrogen Molecular Orbital Diagram Diagram Molecular Orbital Png Pngwing
30:00Molecular Orbital Energy Level Diagram of Ethylene Molecule: 531 views531 views. Feb 27, 2019. 7. 0 ...28 Feb 2019 · Uploaded by LNG CHEMICAL SCIENCES
Solved Draw The Molecular Orbital Diagram For The Excited States Of Butadiene Will It Undergo A 4 2 Cycloaddition With Ethylene Why Or Why Not Show A Picture
Consider the concerted reaction of allyl cation with either ethylene or butadiene! Using Valence Bond Theory, the reaction should occur with either ethylene or butadiene! Using Molecular Orbital Theory, however, first need to consider the symmetry ! of the interacting orbitals! LUMO ! of allyl cation! HOMO ! of ethylene! Incorrect symmetry to form
In ethylene, each carbon combines with three other atoms rather than four. There is a formation of a sigma bond and a pi bond between two carbon atoms. C2H4 Molecular Geometry And Bond Angles. C2H4 molecular geometry is said to be planar in structure while the sp 2 orbitals are placed at a bond angle of 120 o.
Interactions between Ethylene Molecular Orbitals and Metal d Orbitals. could not be loaded. Script error: Forbidden is not defined. Ethylene is capable of acting as a ligand as the C=C π bond can donate electron density to an empty metal d orbital, forming a σ bond. A filled metal d orbital is capable of donating electron density into the C=C ...
3:26This video shows that the pi framework and sigma framework of ethylene are distinct orbital sets.18 Jan 2010 · Uploaded by jeffrey Moore

Draw The Molecular Orbital Energy Diagram For Oxygen Molecule O2 And Show That I It Has A Double Bond Ii It Has Paramagnetic Character From Chemistry Chemical Bonding And Molecular Structure Class 11 Cbse
Chapter 1 Molecular Orbital Concepts A Concepts Of Mo Theory 1 Strong Covalent Bonds Consider The Pi Bond Of Ethene In Simple Molecular Orbital Terms The Qualitative Results Would Be The Same For Any Pi Or Sigma Bond Q The Overlap Of The Two


















0 Response to "41 ethylene molecular orbital diagram"
Post a Comment