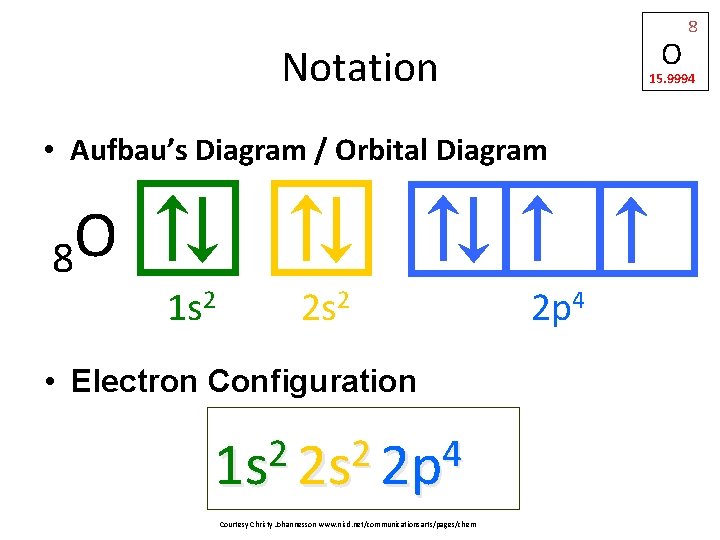
42 orbital diagram for s
required and write a partial orbital diagram. PROBLEM: Use partial orbital diagrams to describe how mixing of the atomic orbitals of the central atom(s) leads to hybrid orbitals in each of the following: (a) Methanol, CH. 3. OH (b) Sulfur tetrafluoride, SF. 4 (a) CH. 3. OH. The electron- group arrangement is tetrahedral around both the C and ... The orbital diagram shows how the electrons are arranged within each sublevel. The maximum number of electrons allowed in an orbital is #2# , each with opposite spins (Pauli';s exclusion principle). In a neutral carbon atom, the #"1s"# sublevel has one orbital with two electrons with opposite spins, represented by the arrows pointing in opposite ...
Orbital diagrams are like the configuration notation just introduced, except with the spins of electrons indicated. Use the Pauli exclusion principle and Hund';s rule to work out how to fill shells. The exclusion principle states that no two electrons can share the same four quantum numbers, which basically results in pairs of states ...

Orbital diagram for s
The S orbital energies are -22.7 eV (3s) and -11.6 eV (3p); the 1s of H has an energy of -13.6 eV. Because of the difference in their atomic orbital energies, the 1s orbital of hydrogen and the 3s orbital of sulfur interact only weakly; this is shown in the diagram by a slight stabilization of orbital diagram for sodium confirms that the 3s sublevel is lower in energy than the 3p sublevel. The s sublevel is located lower on the page than the p sublevel. 10. The lowest potential energy arrangement of electrons in an atom is called the ground state. An orbital diagram illustrates how the electrons pair off in each orbital. Now that you've mastered the world of electron configurations, it';s time to write orbital filling diagrams. This sounds like something that would be tough, but orbital filling diagrams are really just pictures that show you the same thing as electron configurations.
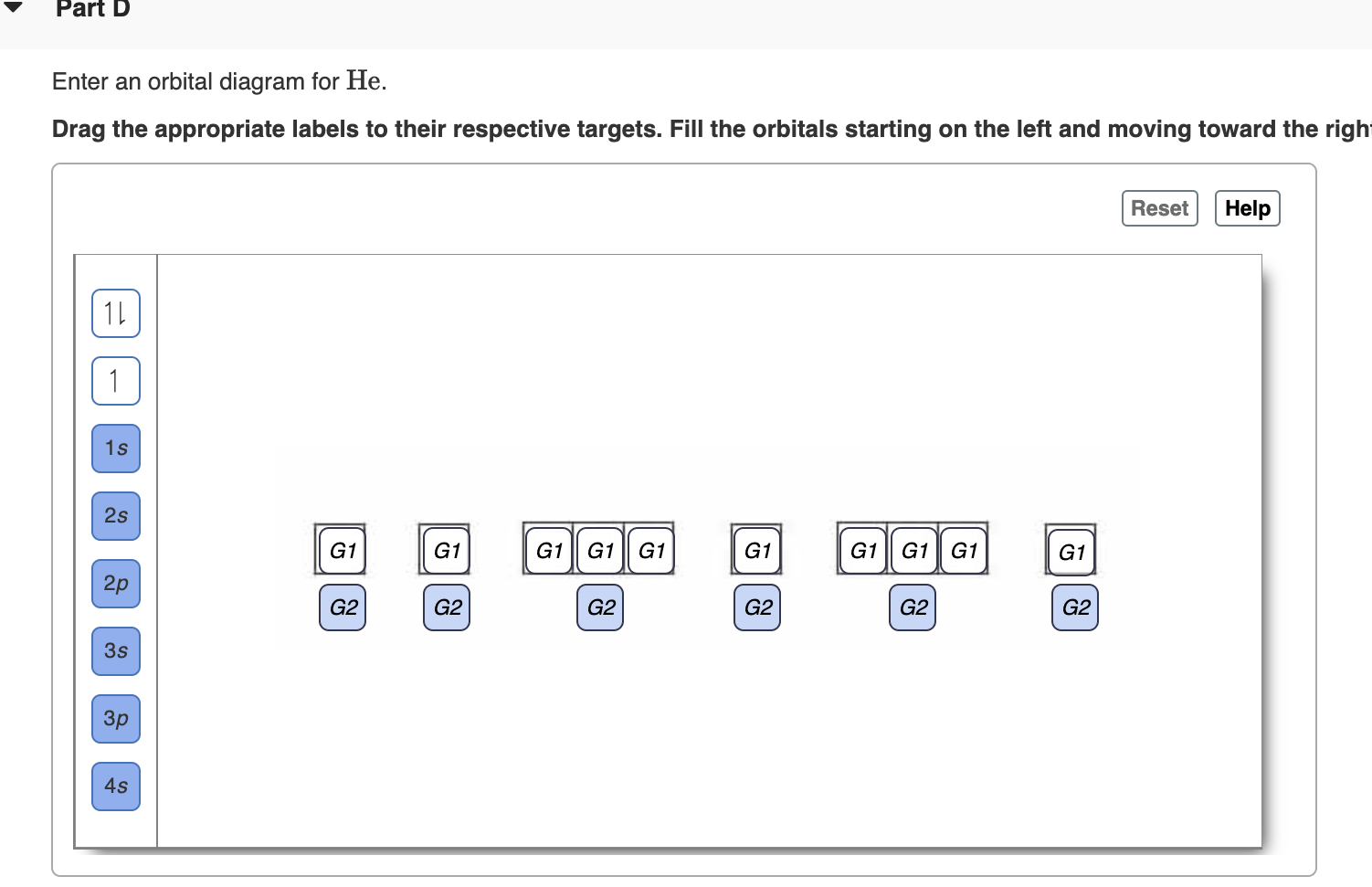
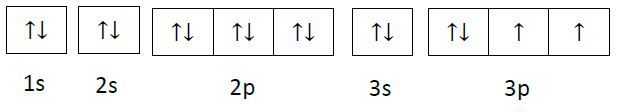
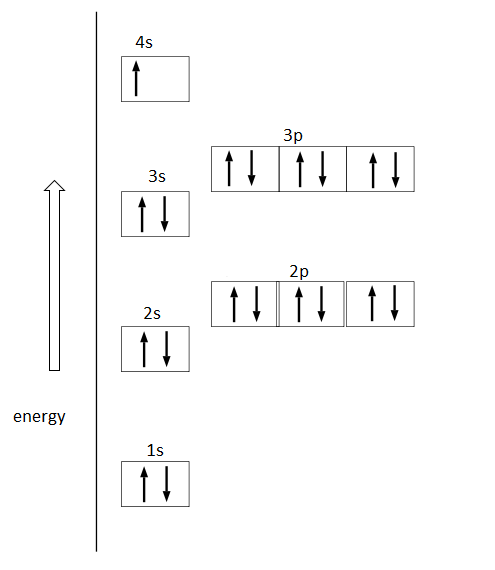
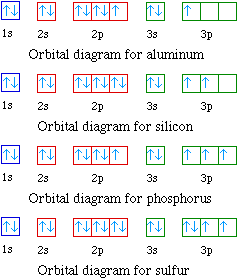
Orbital diagram for s. Fig 2: Orbital diagram. This orbital diagram determines an atom';s electron configuration. Following are some features of this configuration. Each sub-shell can contain a maximum of two electrons. An electron pair means electrons that stay together in a sub-shell or orbital. An electron always enters an orbital having lowest energy. The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each. The arrows represent the 16 electrons of the sulfur atom, and the directions represent their spins. The boxes represent sulfur's orbitals. 26 Jan 2021 — when we the electron configuration of Sulfur the first two electrons go in the 1s orbital. As 1s only hold two electrons and the next two ... Show the orbital-filling diagram for S (sulfur). Order subshells by energy, with the lowest-energy subshell at the left. Drag the appropriate labels to their respective targets. View Available Hint(s) Reset Help 11 1 15 2s 2p 3s 3p G1 G1 G1 G1G1 G1 G1 G1 | G1 G2 G2 G2 G2 G2 Submit Part D Show the orbital-filling diagram for Br (bromine).
A 3s orbital is even larger, and it has three nodes. p ORBITALS. Not all electrons inhabit s orbitals. At the first energy level, the only orbital available to electrons is the 1s orbital. However, at the second level, there are also orbitals called 2p orbitals in addition to the 2s orbital. Sulfur (S) has an atomic mass of 16. Find out about its chemical and physical ... Electron Configuration, [Ne] 3s2 3p4. 1s2 2s2 2p6 3s2 3p4. Orbital Diagram. In writing the electron configuration for Sulfur the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons ...24 Oct 2016 · Uploaded by Wayne Breslyn orbital diagram (orbital box diagram) : Pairs of electrons occupy the 1s, 2s, 2p x, 2p y, 2p z, 3s, 3p x, 3p y, 3p z, 4s orbital and three of the 3d orbitals, with only 1 electron occupying each of the other 3d orbitals and these electrons have parallel spin (arrows pointing in the same direction) in accordance with Hund';s Rule.
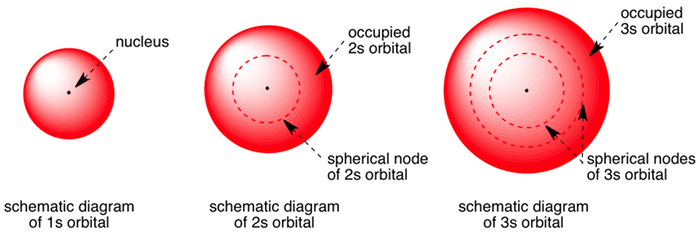
The Shape of s Orbitals. The boundary surface diagram for the s orbital looks like a sphere having the nucleus as its centre which in two dimensions can be seen as a circle. Hence, we can say that s-orbitals are spherically symmetric having the probability of finding the electron at a given distance equal in all the directions. An orbital diagram, or orbital filling diagram, is a type of notation which illustrates an ... S orbitals make up 1 box, and contain a max of 2 electrons ... Orbital diagram of Sulfur (S) 17: Orbital diagram of Chlorine (Cl) 18: Orbital diagram of Argon (Ar) 19: Orbital diagram of Potassium (K) 20: Orbital diagram of Calcium (Ca) 21: Orbital diagram of Scandium (Sc) 22: Orbital diagram of Titanium (Ti) 23: Orbital diagram of Vanadium (V) 24: Orbital diagram of Chromium (Cr) 25: Orbital diagram of ... MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
S Orbital Versus P Orbital . While orbital numbers (e.g., n = 1, 2, 3) indicate the energy level of an electron, the letters (s, p, d, f) describe the orbital shape. The s orbital is a sphere around the atomic nucleus. Within the sphere there are shells in which an electron is more likely to be found at any given time. The smallest sphere is 1s.
s-orbital rotation about the C ∞, and C 2 axes leaves the s-orbital unchanged ∞ C 2 thus the "1" character in the row highlighted for the s-orbital, and under the columns headed C ∞, and ∞C 2 1 -1
Hund©s Rule & Orbital Filling Diagram Complete the orbital diagram for each element. 2) calcium 1s 2s 4s 3s 3d 2p 4p 3p 1) sodium 1s 2s 4s 3s 3d 2p 4p 3p 3) nickel 1s 2s 4s 3s 3d 2p 4p 3p 4) silicon 1s 2s 4s 3s 3d 2p 4p 3p 5) iron 6) copper 1s 2s 4s 3s 3d 2p 4p Answer key 3p 2s2 2p6 3s2 3p6 4s2 1s 2s 4s 3s 2p 4p 3p 3d. Created Date:
What element is represented by this orbital diagram? Pauli Exclusion Principle. 2 electrons in the same orbital must have opposite spins. Hund';s Rule. Electrons don't pair up in orbitals of equal energy until they have to, and all electrons in singly occupied orbitals have the same spin.

Give The Correct Order Of Initials T True F False For Following Satements I If Electron Has Zero Quantum Magnetic Numbers Then It Must Be Present In S Orbital Ii In Img Src Https D10lpgp6xz60nq Cloudfront Net Physics Images
What is the Orbital Diagram For Nitrogen? When we talk about the orbital diagram, we first need to understand what exactly it means. Therefore, during exams, the student can expect questions related to this topic so it is important that the students must go through it.
This video goes over how to properly draw orbital diagrams for an element, after determining the electron configuration. You will also learn how to use hund'...
- The SHAPE of an orbital is defined by the SUBSHELL it is in - The ENERGY of an orbital is defined by both the SHELL the orbital is in AND the kind of SUBSHELL it is in ARRANGEMENT OF SHELLS, SUBSHELLS, AND ORBITALS - Shells are numbered. Each shell can contain the same number of SUBSHELLS as its number: 1st shell: ONE possible subshell (s)
Show the orbital-filling diagram for (bromine).Status: Resolved. Show the orbital-filling diagram for S (sulfur). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top%(15). 1. Describe the two differences between a 2p x orbital and a 3p y orbital.
According to Hund';s rule, as electrons are added to a set of orbitals of equal energy, one electron enters each orbital before any orbital receives a second electron. Orbital Filling Diagrams An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom.
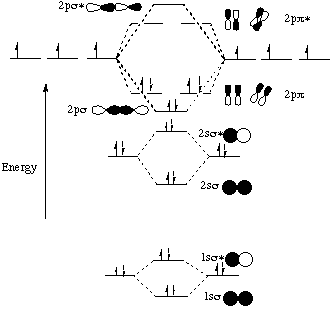
Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2? Solution. We draw a molecular orbital energy diagram similar to that shown in Figure 11.

Atomic Orbital Electron Configuration Molecular Orbital Diagram Iron Ferric Iron Angle White Electronics Png Pngwing
Diagram of the S and P orbitals The s subshells are shaped like spheres. Both the 1n and 2n principal shells have an s orbital, but the size of the sphere is larger in the 2n orbital. Each sphere is a single orbital. p subshells are made up of three dumbbell-shaped orbitals. Principal shell 2n has a p subshell, but shell 1 does not.
Orbital diagrams are a visual way to show where the electrons are located within an atom. Orbital diagrams must follow 3 rules: The Aufbau principle, the Pau...

Which Of The Orbital Diagrams Represent S An Excited State Nitrogen Atom Choose One Or More 1 2p Homeworklib
An sp hybridized atom uses one s and one p orbital to make two sp hybrid orbitals; there are two remaining p orbitals. Next we show the phase pictures of combining the sp hybrid orbitals with fi rst one and then both of the remaining p orbitals. Phase pictures. loop Diagram Axes for you to draw the loop diagram.
26 Apr 2021 — An electron configuration lists only the first two quantum ... has a single electron in the outer s-orbital: H 1s1, Li 2s1, Na 3s1 , K 4s1.
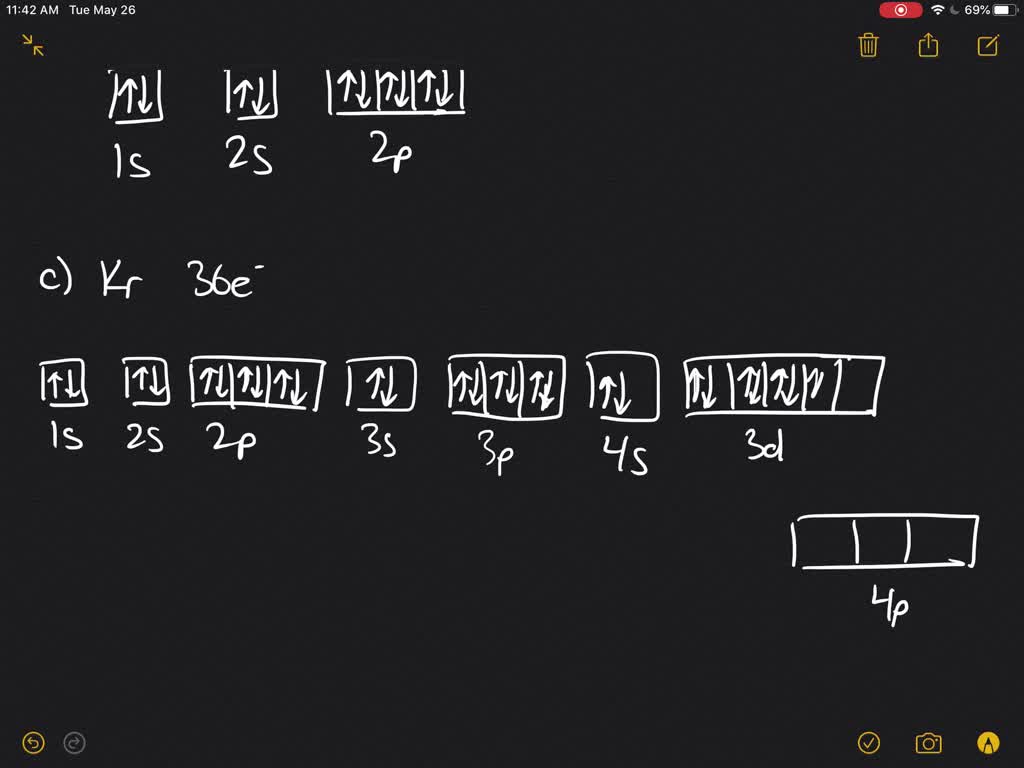
Solved Write The Complete Orbital Diagram For Each Of The Following Elements Using Boxes To Represent Orbitals And Arrows To Represent Electrons Begin Array Ll Text A Helium Z 2 Text C Krypton
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ...
An orbital diagram illustrates how the electrons pair off in each orbital. Now that you've mastered the world of electron configurations, it';s time to write orbital filling diagrams. This sounds like something that would be tough, but orbital filling diagrams are really just pictures that show you the same thing as electron configurations.
orbital diagram for sodium confirms that the 3s sublevel is lower in energy than the 3p sublevel. The s sublevel is located lower on the page than the p sublevel. 10. The lowest potential energy arrangement of electrons in an atom is called the ground state.
The S orbital energies are -22.7 eV (3s) and -11.6 eV (3p); the 1s of H has an energy of -13.6 eV. Because of the difference in their atomic orbital energies, the 1s orbital of hydrogen and the 3s orbital of sulfur interact only weakly; this is shown in the diagram by a slight stabilization of

Palladium Pd Z 46 Is Diamagnetic Draw Partial Orbital Diagrams To Show Which Of The Following Brainly Com
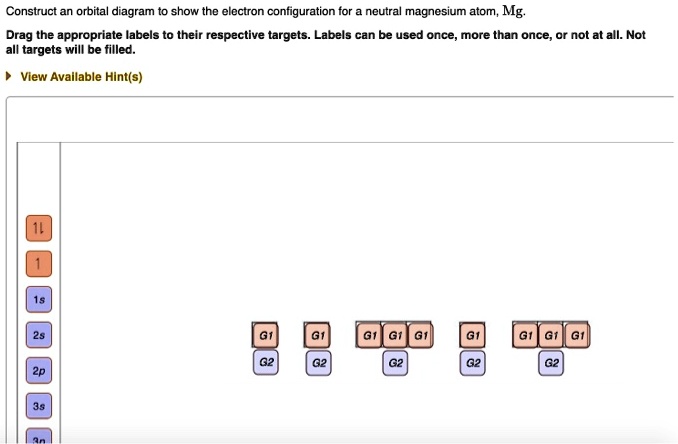
Solved Construct An Orbital Diagram To Show The Lectron Configuration For Neutral Magnesium Atom Mg Drag The Appropriate Labels To Their Respective Targets Labels Can Be Used Once More Than Once Or Not
Quantum Numbers Electron Configuration And Orbital Diagrams Small Online Class For Ages 11 16 Outschool














0 Response to "42 orbital diagram for s"
Post a Comment