37 li2+ molecular orbital diagram
Keywords: MO diagram, C22+, B22-, Li2-, molecular orbital diagram, paramagnetic, diamagnetic, paramagnetism, diamagnetism, paired electrons, unpaired electrons. Answer : For a species to be diamagnetic it needs to have no unpaired electrons and for paramagnetic the species needs t have unpaired electrons.
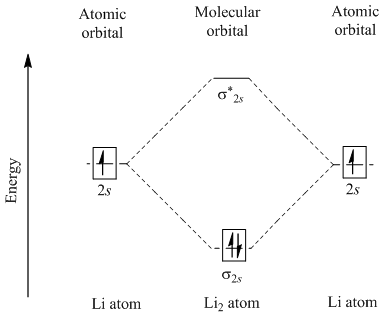
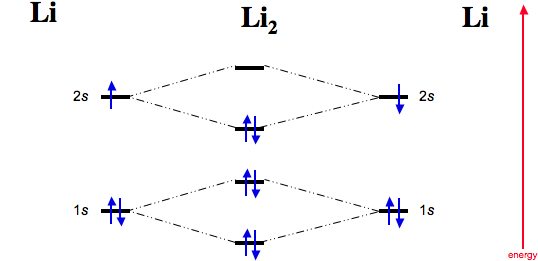
Li2 has all of its electrons neatly paired up in the orbitals. Li2 is diamagnetic. Explore further detail here. In this regard, what is the bond order of li2 −? The 1s electrons do not take part in the bonding, but the 2s electrons fill the bonding orbital. The molecule Li2 is a stable molecule in the gas phase, with a bond order of one.
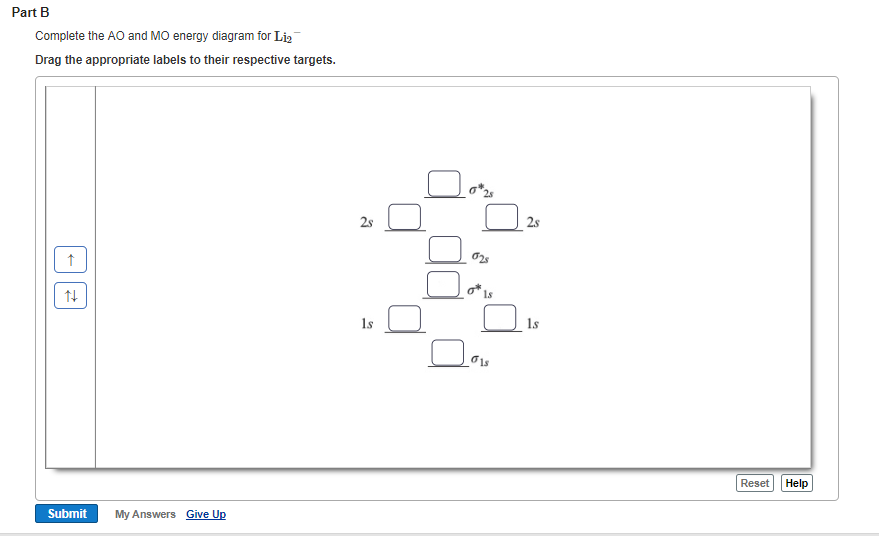
Chemistry. Chemistry questions and answers. Construct the molecular orbital diagram for Li2. Note that the 1s orbitals are not shown. Li Li Li Answer Bank 2s 2s、、 02s Identify the bond order O 0 O 05 O 1 O 1S 02.5. Question: Construct the molecular orbital diagram for Li2. Note that the 1s orbitals are not shown.

Li2+ molecular orbital diagram
Answer: This is a very ambitious calculation. F. A. Matsen did such a calculation on the molecule Li-H about fifty years ago, but the calculations have become more streamlined since then. The results from such calculations will not actually predict the "existence" of Li2C2, but will provide a re...
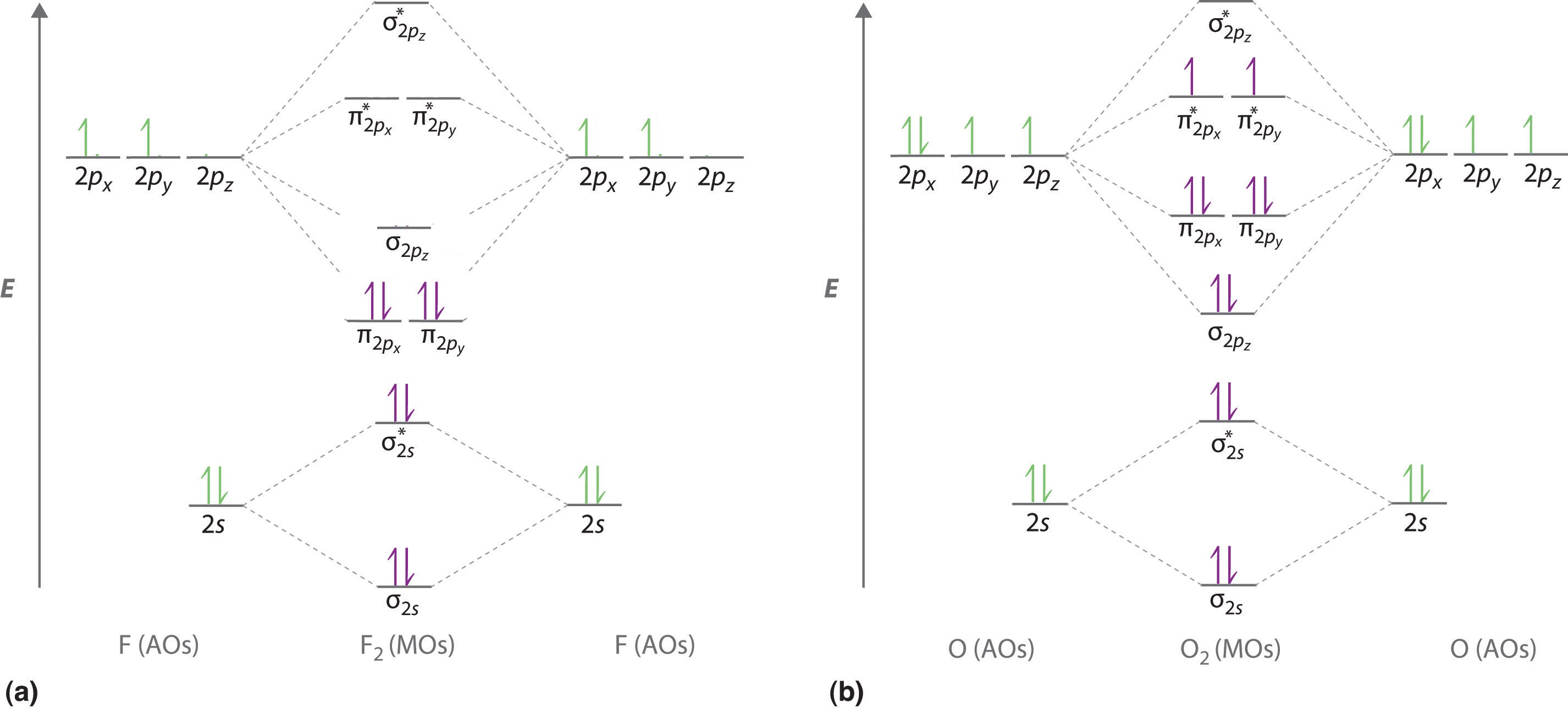
Printable O2 molecular orbital diagrams are available for you to guide your study in the molecular orbital lesson.This diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of a molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.
Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in
Li2+ molecular orbital diagram.
Get access to the latest Bonding in Homonuclear Diatomic Molecules: Li2, Li2+, Be2, B2, C2, N2 prepared with IIT JEE course curated by Megha Khandelwal on Unacademy to prepare for the toughest competitive exam.
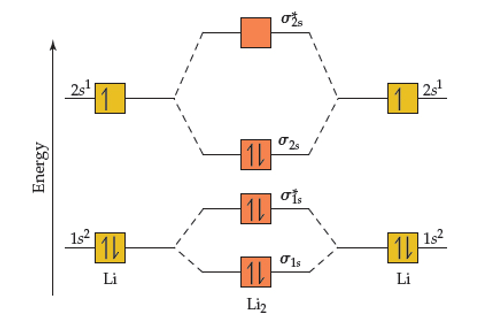
Molecular orbital electronic configuration of Li₂- : σ1s² σ*1s²σ2s2 σ*2s¹, bond order = (Nb - Na)/2 = (4-3)/2 = 0.5. As the bond orders are same in both the species, it is expected that their bond lengths are same. But as more electrons in anti bonding orbital lengthens the bond, the bond length of Li₂- is slightly greater than that of Li₂+.
This chemistry video tutorial provides a basic introduction into molecular orbital theory. It describes the formation of bonding and antibonding molecular o...
Molecular orbital theory : As long as the specific molecular orbitals forms (their dependency on R and Z in the cylindrical coordination system) vary for each and every molecule, their dependency on an angle 'f' due to the represented by quantum number L and their behavior of G or U w.r.t to inversion are entirely determined by system's geometry.
Dilithium, Li 2, is a strongly electrophilic, diatomic molecule comprising two lithium atoms covalently bonded together. Li 2 is known in the gas phase.It has a bond order of 1, an internuclear separation of 267.3 pm and a bond energy of 102 kJ/mol or 1.06eV in each bond. The electron configuration of Li 2 may be written as σ 2.. It has been observed that 1% (by mass) of lithium in the vapor ...
Molecular structure: Born-Oppenheimer approximation, molecular orbital ... As the atomic number increases from three for lithium to ... ++ ** ++ -ftft.* **.23 pages
(1986). These measurements were performed at much higher resolution, 0.002–0.004eV FWHM, and displayed sharp structure obscured in the (e,e) data of Daviel ...
Molecular orbital diagram of Li2 & Be2: Number of electrons in Li2 molecule =6. Li2 = σ1s2,σ*1s2,σ2s2. Nb=4, Na=2. B.O = (Nb- Na). B.O = (). B.O = 1. Explain why the relative energy levels diagram s for Li2, Be2, B2, C2, N2 are different The molecular orbital theory of Li2 to F2 gives a graphical explanation.
Molecular orbital theory describes the distribution of electrons in molecules in much the same way that the distribution of electrons in atoms is described using atomic orbitals. Using quantum mechanics, the behavior of an electron in a molecule is still described by a wave function, Ψ, analogous to the behavior in an atom.Just like electrons around isolated atoms, electrons around atoms in ...
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining . However, experimental and computational results for homonuclear diatomics from Li2 to N2 and certain heteronuclear combinations such as CO and NO. Molecular orbital diagram of Li2 & Be2: Number of electrons in Li2 molecule =6. Li2 = σ1s2,σ*1s2,σ2s2.
The valence molecular orbital diagram for li2 is shown. It has 882 18 electrons. Molecular electron configuration for o2 σ2σ2σ2π4π2 we can also calculate the oo bond order. Practice energy diagrams for molecular orbital theory. The molecular orbital bond order for this species is equal to and li2 is stable than li2 12.
Transcribed image text: Consider the Molecular Orbital Interaction Diagram for Li2. Which of the following pictures shows the atomic orbitals on the separate atoms that combine to make the LUMO in Liz? The shading represents the phases of the wavefunction. o S + S o S + S o p + P o 88 88 p + p O p + p o р + р Consider the Molecular Orbital Interaction Diagram for N2.
++. -. +. -. +. -. Walsh's correlation diagram: Plot course of MO energies with change in geometry. Chem 104A, UC, Berkeley. Walsh's Rules:.32 pagesMissing: li2 | Must include: li2
Catherine E. Housecroft, Edwin C. Constable · 2010 · Science2s + + 2s ++ 0 ( 25 ) Li Li2 Li However , of the six electrons in the Li2 ... Thus , MO theory predicts that the Li2 molecule possesses two electrons which ...
How to draw the molecular orbital diagram of Li2 , Li2 + , Li2 - ? Incase of Li 2 + , the 1 electrons are present in σ 2s orbitals . The anti bonding molecular orbital σ*2 s is empty . Thus Number of electrons in BMO - Number of electrons in ABMO 1- Bond order = 0.5 Similarly, Incase of Li 2 - , the 2 electrons are present in σ 2s orbitals .
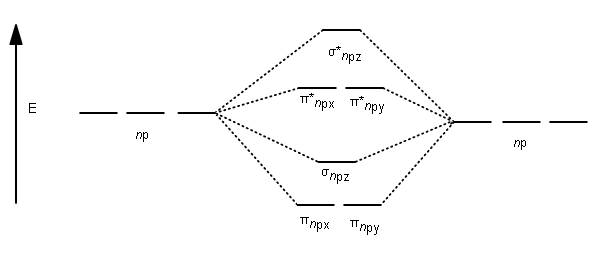
Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ...
Which of the following is paramagnetic? (use the molecular orbital diagram) a) Li 2. b) Be 2. c) B 2. d) C 2. e) N 2. Learn this topic by watching MO Theory: Homonuclear Diatomic Molecules Concept Videos.
Use of molecular orbital theory facilitates an understanding of physical properties ... 1s AO on hydrogen, x1, and the 2s AO on lithium, x2.831 pages
How to draw Molecular Orbital Diagram of Li2 ,Li 2+ , Li2 - | Simplest Trick - Chemistry By anumsunum on August 20, 2021 • ( Leave a comment ) Also Watch Molecular orbital diagram of O2 , O2 +2 , 02 - 2 ( in Urdu / Hindi)
11+ Li2 Molecular Orbital Diagram. This chemistry video tutorial provides a basic introduction into molecular orbital theory. (a) the diagram for h2, he2, li2, be2, b2, c2, and n2. Relationship between electronic configuration and molecular behaviour. Number of electrons in c2 molecule = 12. Source: d2vlcm61l7u1fs.cloudfront.net
The molecular orbital electronic configuration,Magnetic property: Since bond order is zero, Be2 molecule does not exist. It is diamagnetic due to the ... Rating: 4.4 · 740 votes · Free · Android · EducationalMissing: ++ | Must include: ++
a blank molecular orbital diagram (part b 1 figure) has been provided to you. drag the formulas to the appropriate magnetic bin : c2^2+,li2-,b2^2- Answers: 1
Molecular orbital energy level of Li2. diagram; the MOs go between them. Consider the MO diagram for Li2. This is a bond between two lithium atoms, which have an electron configuration of. The last diagram presents the molecule dilithium (Li2). The 1s electrons do not take part in the bonding, but the 2s electrons fill the bonding orbital.
Molecular orbital energy level of Li2. MO diagrams for Diatomic Molcules. Overview. In this section, we will compare MO diagrams for diatomic molecules X-X, from Li2 to Ne2. Molecular orbital diagram of Li2 & Be2: Number of electrons in Li2 molecule =6. Li2 = σ1s2,σ*1s2,σ2s2. Nb=4, Na=2. B.O = (Nb- Na). B.O = (). B.O = 1.
Full first shell:sigma-1s and sigma1s* are both full.sigma-2s is full; sigma-2s* is empty.Bond order 1 = single bond.Check me out: http://www.chemistnate.com
Li2 is more stable than Li+ 2, because the bond is (hypothetically) stronger (probably gas-phase). Here we consider the molecular orbital diagram (MO) of Li2: The bond order can be calculated in a simple manner. Just take electrons that are in each MO, and. for each electron in a bonding MO, it adds 0.5 to the bond order, because more bonding ...
This is the molecular orbital diagram for the homonuclear diatomic Be2+, . electrons would be in a bonding orbital, we would predict the Li2 molecule to be . Explain why the relative energy levels diagrams for Li2, Be2, B2, C2, N2 are different The molecular orbital theory of Li2 to F2 gives a graphical explanation.
Figure 13: A molecular orbital energy-level diagram showing the relative energies of the atomic orbitals of atoms A and B (1sA and 1sB) and the bonding (1σ) and ...Missing: ++ | Must include: ++
molecular orbitals in the diagram suggest a double bond. c. The 2s, 2s *, 2p, and 2p * orbitals exhibit C v symmetry, with the NF bond axis the infinite-fold rotation axis. The 2p and 2p * orbitals exhibit Cs symmetry. The latter do not possess C2 rotation axes coincident to the
The last diagram presents the molecule dilithium (Li2). The 1s electrons do not take part in the bonding, but the 2s electrons fill the bonding orbital. The molecule . Molecular orbital diagram of Li2 & Be2: Number of electrons in Li2 molecule =6. Li2 = σ1s2,σ*1s2,σ2s2. Nb=4, Na=2. B.O = (Nb- Na). B.O = (). B.O = 1.
























0 Response to "37 li2+ molecular orbital diagram"
Post a Comment