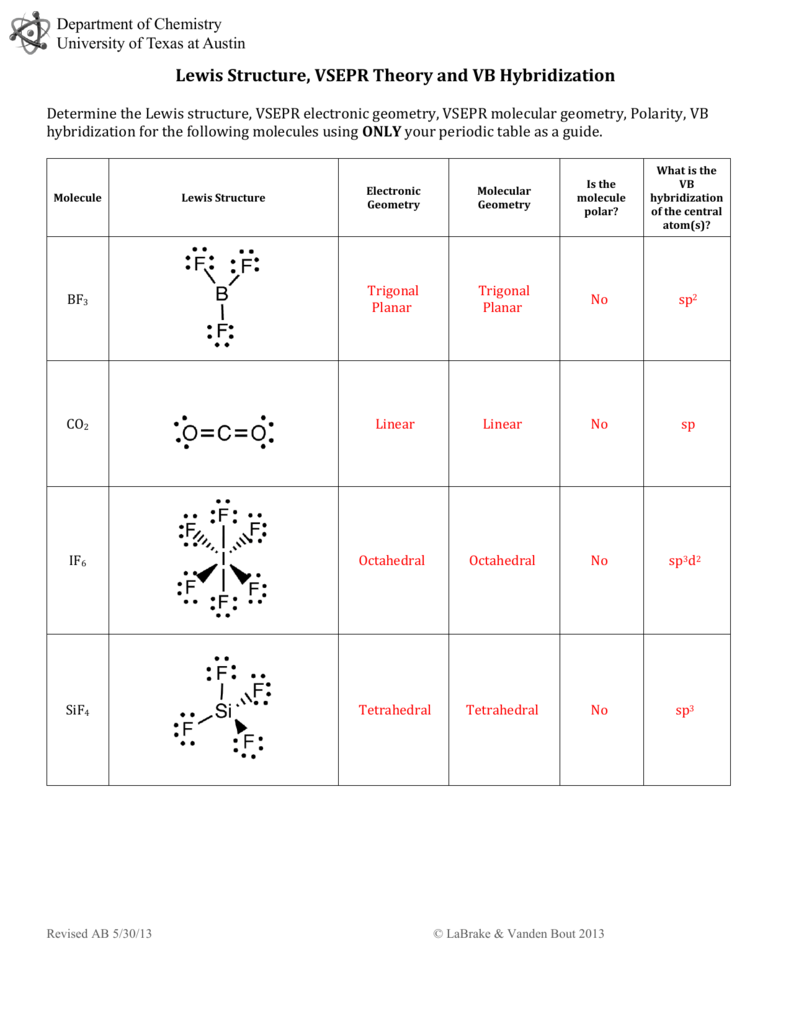
38 lewis diagram for bf3
Drawing the Lewis Structure for BF 3. Video: Drawing the Lewis Structure for BF 3. For the BF 3 Lewis structure, calculate the total number of valence electrons for the BF 3 molecule. There are a total of 24 valence electrons for the BF 3 Lewis structure. After determining how many valence electrons there are in BF 3, place them around the central atom to complete the octets. Lewis Dot Structure For Boron Trifluoride, BF3 Lewis Structure: How to Draw the Lewis Structure fo, BF3 Lewis Structure, Molecular Geometry, Hybridization, Lewis Diagram For Bf3 General Wiring Diagram, Boron trifluoride Alchetron, The Free Social Encyclopedia
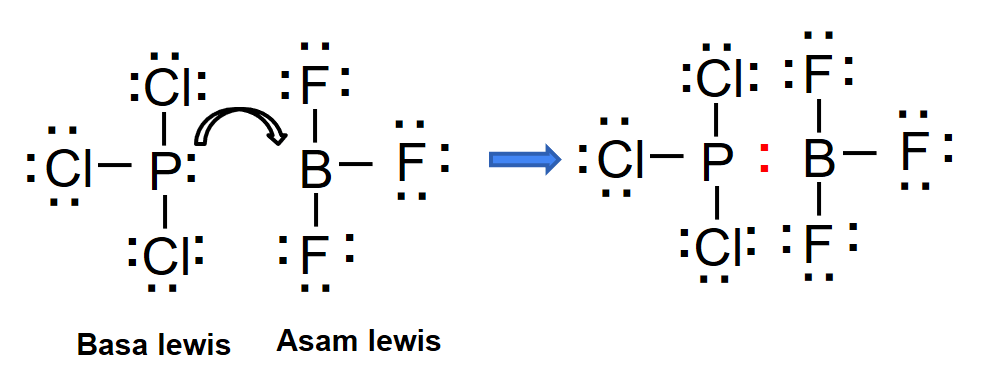
In the reaction BF 3 + F - --> BF 4 - the BF 3 is acting as a lewis acid in the reaction beacuse a lewis acid is one that accepts a pair of electron.. normally lewis acids are species with vacant orbitals,while lewis bases are species with lone pair of electron , in the given reaction the BF3 has a vacant orbital ( draw the lewis structure of BF3) while F- has the lone pair of electron which ...
Lewis diagram for bf3
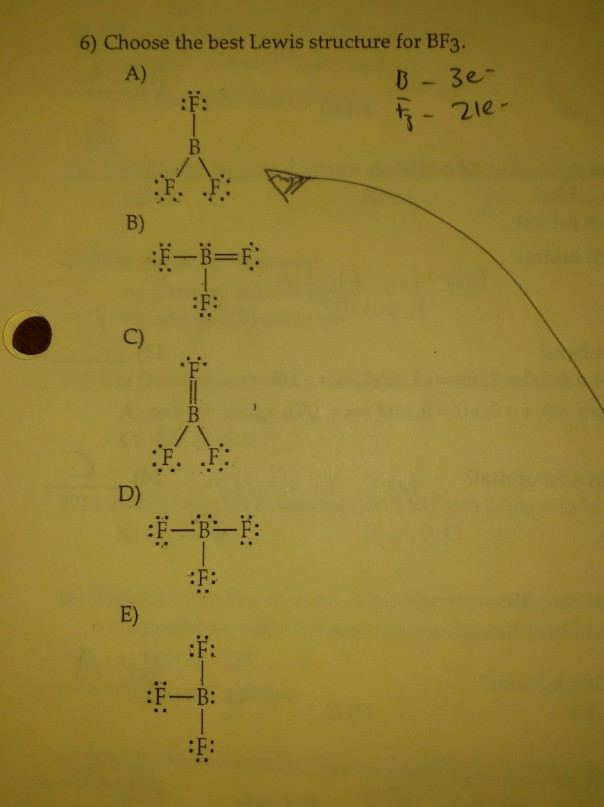
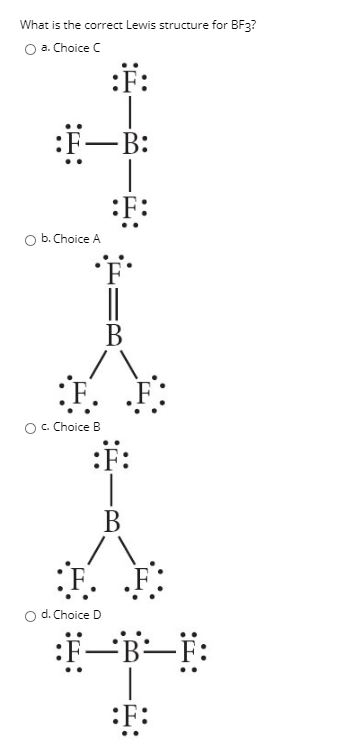
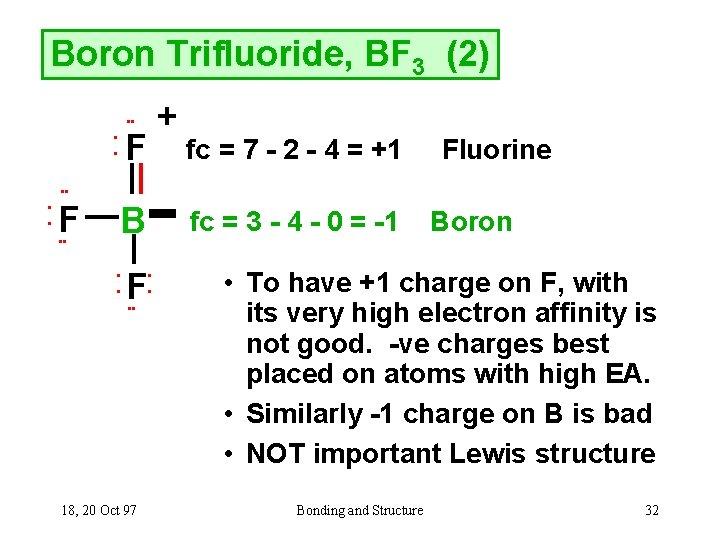
Problem: For the molecule BF3, if we write the Lewis dot structure by first completing the octet around the pendant F atoms, we find that we have used all the valence electrons but the B has less than an octet (Structure A). Using our rules for drawing Lewis dot structures, we complete the octet around the B by forming double bonds from one of the F atoms which give rise to the resonance ... Hydrogen is usually surrounded by 4 electrons in a valid lewis structure. Bf3 has a total of 24 valence electrons, which we have to set around the central atom. Lewis structure, then the molecular geometry of the molecules. Ax 2 has linear shape. Predicted data is generated using the us environmental protection agency's episuite™. BF3, boron trifluoride, is a tricky molecule to draw because Boron is an exception to the octet rule.It does not need eight electrons in its outer shell, although it can hold eight just like most other non-metals.. The Lewis Structure of BF3, boron trifluoride, has one boron atom in the centre, and three fluorine atoms surrounding it.
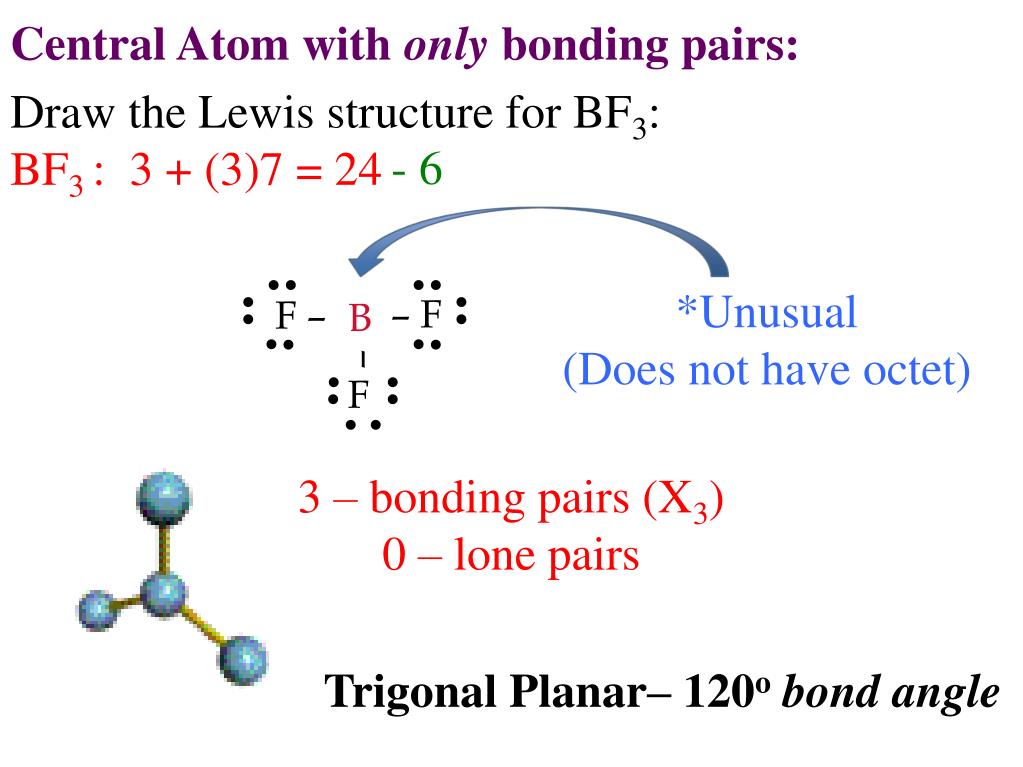
Lewis diagram for bf3. Lewis Diagram and Molecular Shape of Nitrogen trifluoride NF3 Lewis Diagrams and Molecular Shape of Boron trifluoride BF3 Be sure to subscribe to my Youtube Channel! BF3 Lewis Structure To know the structure BF3 Lewis, we need to calculate the total number of valence electrons for the BF3 molecule. BF3 has a total of 24 valence electrons, we must set around the central atom. Before completing octets, do not forget to determine how many valence electrons there in Boron trifluoride and place them accordingly. BF3 Lewis Structure. To know about BF3 Lewis structure, we have to calculate the total number of valence electrons for the BF3 molecule. BF3 has a total of 24 valence electrons, which we have to set around the central atom. The answer to the question "Is BF3 polar or nonpolar?" is BF3 is non-polar. Boron trifluoride (BF3) is a non-polar inorganic chemical compound. There are a total of 24 valence electrons for the BrF 3 Lewis structure; BF3 molecular geometry is trigonal planer. Boron trifluoride is a colourless gas with a terrible and stifling odour. Related ...
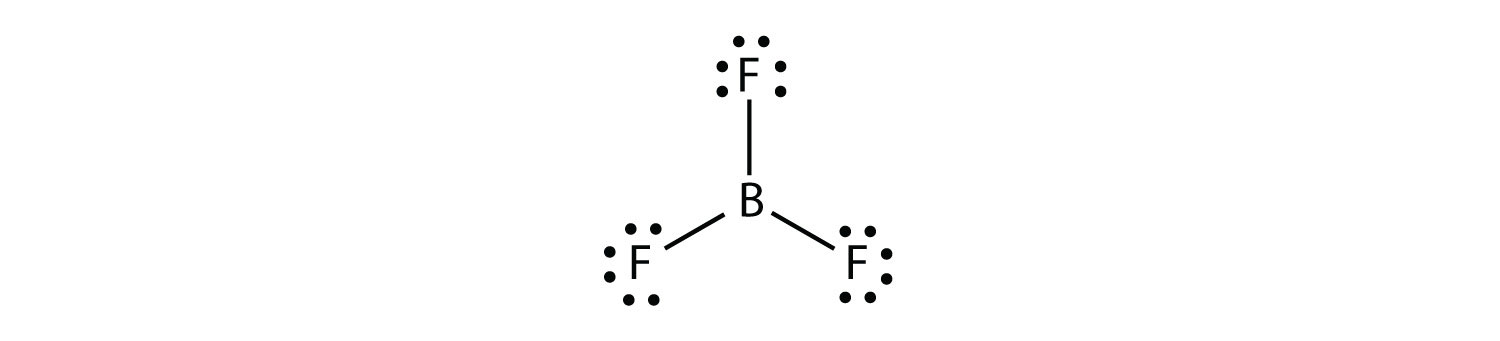
BF3 Lewis Structure, Molecular Geometry, and Hybridization. Boron Trifluoride (BF3) is an inorganic compound as it lacks a carbon atom or C-H bond in the molecule. Manufactured from the reaction of boron oxides and hydrogen fluoride, the chemical compound BF3 has a pungent smell and is colorless in nature. The compound behaves differently in ... A step-by-step explanation of how to draw the BF3 Lewis Dot Structure (Boron Trifluoride).For the BF3 Lewis structure, calculate the total number of valence ... BrF3 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram BrF3, known as Bromine Trifluoride, is a fuming liquid consisting of inter-halogen combinations and bearing a pungent smell. Having a straw i.e, colorless to yellow appearance, this chemical compound has several applications but also comes with a number of limitations and ... There are a total of 24 valence electrons for the BF3 Lewis structure. After determining how many valence electrons there are in BF3, place them around the central atom to complete the octets. Boron is the least electronegative atom in the BF3 Lewis structure and therefore goes at the center of the structure.
Drawing the Lewis Structure for BF 3. Viewing Notes: The BF 3 Lewis structure is similar to BCl 3 and BBr 3 since Cl and Br are in Group 7 and have 7 valence electrons.; Boron (B) doesn't need 8 valence electrons to have an octet (Boron often only needs 6). If you're not sure you have the best Lewis structure for BF 3 you can calculate the formal charges. You'll find the B in BF 3 only has 6 ... Drawing the Lewis Structure for BrF 3. Video: Drawing the Lewis Structure for BrF 3. For the BrF 3 Lewis structure, calculate the total number of valence electrons for the BrF 3 molecule. There are a total of 28 valence electrons for the BrF 3 Lewis structure. After determining how many valence electrons there are in BrF 3, place them around the central atom to complete the octets. Boron trifluoride is a versatile Lewis acid that forms adducts with such Lewis bases as fluoride and ethers : CsF + BF 3 → CsBF 4. O (C 2 H 5) 2 + BF 3 → BF 3 ·O (C 2 H 5) 2. Tetrafluoroborate salts are commonly employed as non-coordinating anions. 2. how to write the correct Lewis diagram for NH3 and derive the angle between any two H atoms and the N atom. Also explain the direction of; Question: 1. how to write the correct Lewis diagram for BF3 and derive the angle between any two F atoms and the B atom from the diagram. Also explain why the arrows that indicate the bond moments point ...
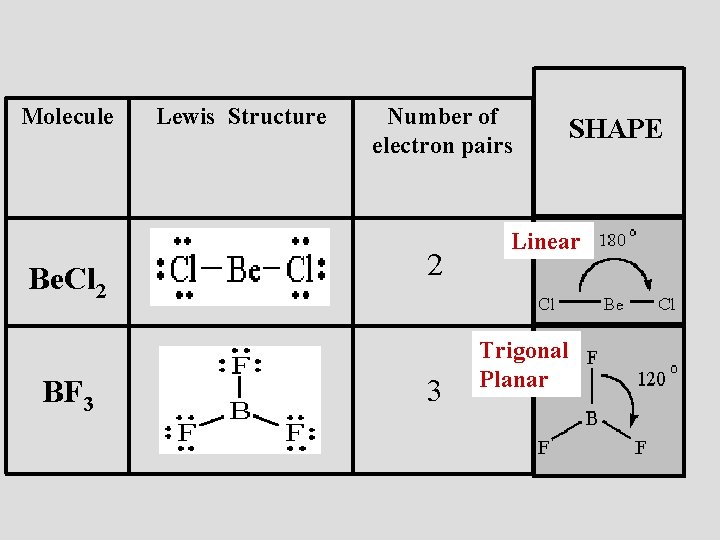
Step 1: Use lewis structure guidelines to draw the lewis structure of BF 3. Step2: Apply VSEPR notation, A X E A=Number of central atoms X=Number of surrounding atoms E= Number of lone pairs on central atom For the above molecule VSEPR notation will be AX 3 E 0. Step 3: Use VSEPR table to find the shape. AX 3 has trigonal planar shape.
Bf3 Molecular orbital Diagram - Bf3 Molecular orbital Diagram , D3h Boron Trifluoride is Loaded. UNTPIKAPPS. Home; ... hybridization and molecular orbital mo theory molecular shapes based on valence electrons lewis dot structures and electron repulsions •molecular orbital theory mo - a molecule is formed by the overlap of atomic orbitals to ...
Draw The Lewis Structure For Bf3 Including Lone Pairs. Xef2o Lewis Structure With Lone Pairs. Bf3 Lewis Structure Molecular Geometry Hybridization And Polarity. Bf3 Lewis Structure Molecular Geometry And Hybridization Techiescientist. Lewis Structure Of Boron Trifluoride Bf3.
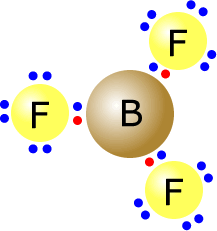
Lewis Structure of Boron Trifluoride (BF. 3. ) Boron trifluoride contains one boron atom and three fluorine atoms. Lewis structure of boron trifluoride (BF 3) is shown below and you can see each fluorine atom has made a single bond with boron atom. Boron atom is the center atom. In this tutorial, we will learn how to draw the lewis structure of ...
Boron trifluoride is a colorless gas with a pungent odor. It is toxic by inhalation. It is soluble in water and slowly hydrolyzed by cold water to give off hydrofluoric acid, a corrosive material.Its vapors are heavier than air. Prolonged exposure of the containers to fire or heat may result in their violent rupturing and rocketing.
What is Lewis structure of bf3? There are a total of 24 valence electrons for the BF3 Lewis structure. After determining how many valence electrons there are in BF3, place them around the central atom to complete the octets. Boron is the least electronegative atom in the BF3 Lewis structure and therefore goes at the center of the structure.
Check me out: http://www.chemistnate.com
Lewis diagrams. Drawing Lewis diagrams. Worked example: Lewis diagram of formaldehyde (CH₂O) Worked example: Lewis diagram of the cyanide ion (CN⁻) Exceptions to the octet rule. Worked example: Lewis diagram of xenon difluoride (XeF₂) Practice: Lewis diagrams. This is the currently selected item. Next lesson.
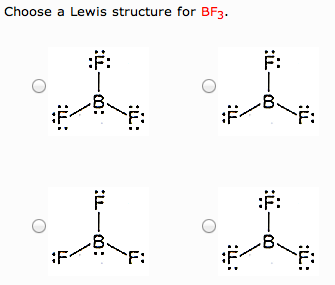
What is the Lewis Structure for BF3, the steric number, the electron pair geometry, the molecular geometry, and how many double bonds does it have? close. Start your trial now! First week only $4.99! arrow_forward. Question.
Ionic (electrovalent), Covalent, Lewis structure, exception octet rule, overlapping hybridisation of the s and p orbitals, shapes of and bond angles, principle of valence shell electron pair repulsion, linear, trigonal planar, tetrahedral, trigonal bipyramid, octahedral, V-shaped, T-shaped, seesaw and pyramidal, polar and non-polar bonds, inertness of nitrogen, Metallic, Intermolecular forces ...
BF3, boron trifluoride, is a tricky molecule to draw because Boron is an exception to the octet rule.It does not need eight electrons in its outer shell, although it can hold eight just like most other non-metals.. The Lewis Structure of BF3, boron trifluoride, has one boron atom in the centre, and three fluorine atoms surrounding it.
Hydrogen is usually surrounded by 4 electrons in a valid lewis structure. Bf3 has a total of 24 valence electrons, which we have to set around the central atom. Lewis structure, then the molecular geometry of the molecules. Ax 2 has linear shape. Predicted data is generated using the us environmental protection agency's episuite™.
Problem: For the molecule BF3, if we write the Lewis dot structure by first completing the octet around the pendant F atoms, we find that we have used all the valence electrons but the B has less than an octet (Structure A). Using our rules for drawing Lewis dot structures, we complete the octet around the B by forming double bonds from one of the F atoms which give rise to the resonance ...
















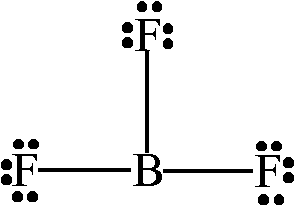



0 Response to "38 lewis diagram for bf3"
Post a Comment