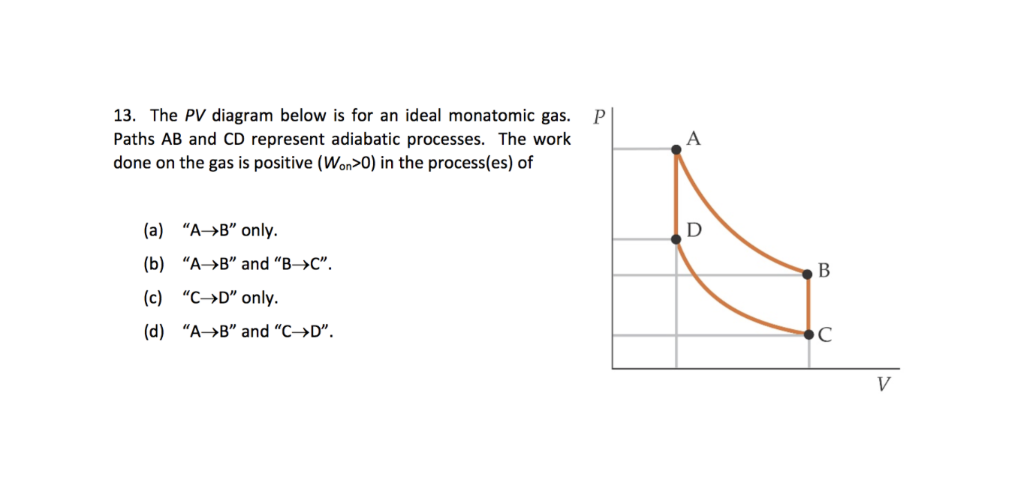
39 adiabatic process pv diagram
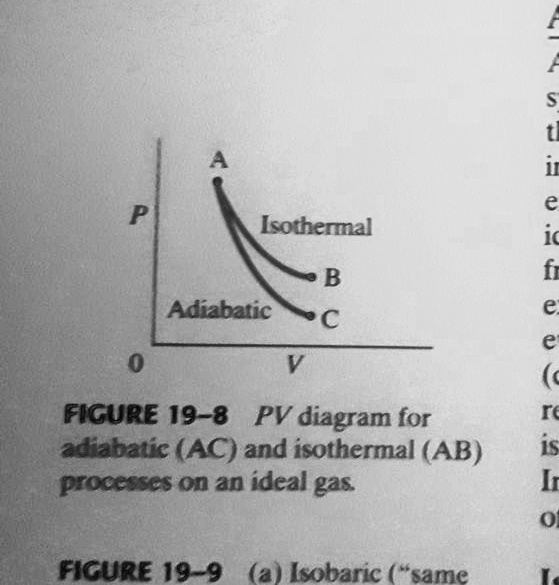
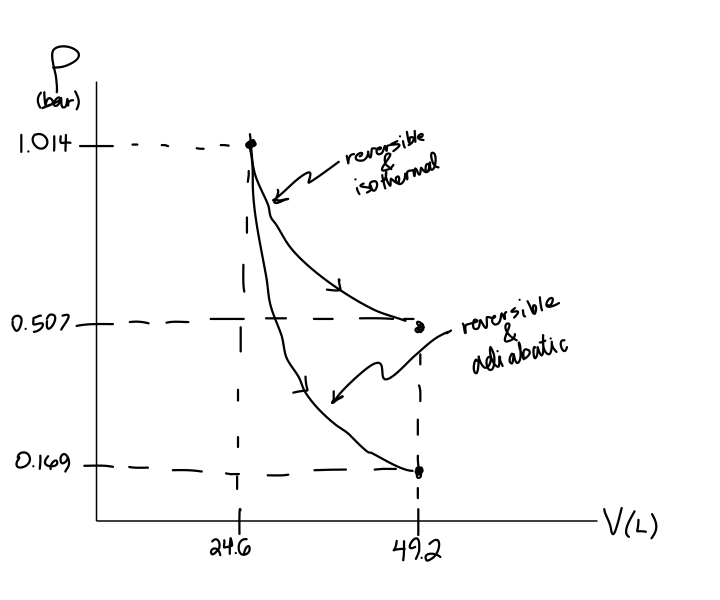
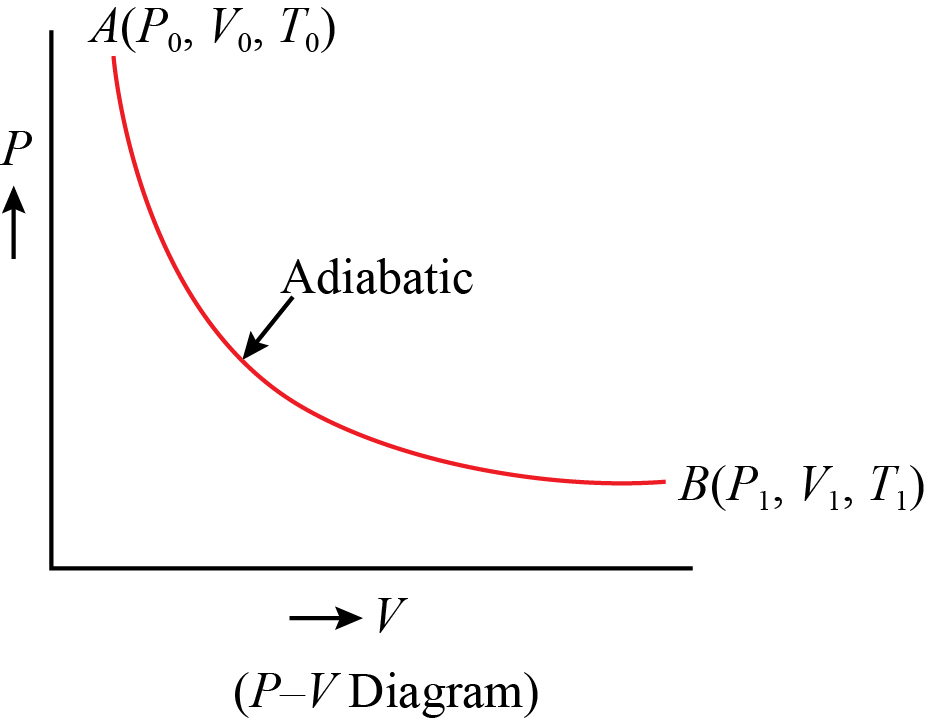
Could someone show me what my PV diagram should look like for the following scenario: An ideal gas, at temp 395 K and Volume 2m^3, expands adiabatically to a V of 4 m^3, then expands isothermically (I believe he meant isothermally) to a V= 10m^3, then compresses adiabatically until its temperature is again 395K. I'm struggling hard with drawing this correctly. Would the 3rd process end up back where the first one started? However, the former exist only as theoretical tools to study the latter. Thus, reversible adiabatic processes involve ideal gases, and lack friction and any other eventuality that causes heat transfer between the system and its surroundings. Consider for example the PV diagram for the reversible adiabatic process above.
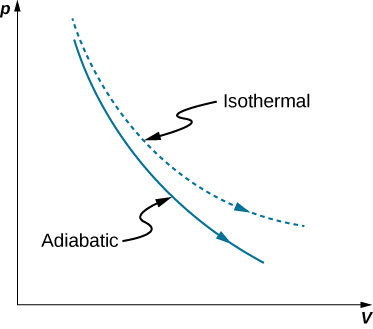
Adiabatic. Adiabatic processes cause an change in internal energy without transfer of heat, but purely through work. An example of a PV diagram and an Energy-Interaction diagram is shown below. Figure 4.4.6: Example of an Adiabatic Process. Adiabatic processes typically occur very quickly, such that the system has not time to exchange heat with its environment. Every time you open a carbonated ...
Adiabatic process pv diagram
The internal energy of systems reduces, resulting in a temperature drop. The relationship between Pressure and Volume in an adiabatic process with ideal gas is given by, PV = γ = Constant . where γ=Cp/Cv. If an ideal gas changes state adiabatically from (P 1, V 1) to (P 2, V 2): P 1 V 1 =P 2 V 2. PV diagram for the adiabatic is, 1st Law for isochoric, isothermal and adiabatic process. • Temperature Three special ideal gas processes: one of, W or Q is 0. • fix volume by 0 for isobaric. Explain the differences among the simple thermodynamic processes—isobaric, isochoric, isothermal, and adiabatic. Calculate total work done in a cyclical. The above diagram represents a graph between temperature T and entropy S for a reversible Carnot cycle. Like the PV diagram, the processes for a TS diagram of a complete Carnot cycle is similar, such as, Line AB = Isothermal expansion, where the temperature T1 = Constant. Line BC = Adiabatic expansion, where the Entropy S2 = Constant.
Adiabatic process pv diagram. Dual cycle is the air standard cycle which is combination of the otto cycle & diesel cycle. It is consists of the two isenthalpic (constant volume) process & two adiabatic process & one isobaric process (Constant volume). (a) The pV-diagram which shows a cycle of a heat engine that uses 0.250 mol of an ideal gas with g = 1.40. Process ab is adiabatic. (i) Find the pressure of the gas at point a. (ii) How much heat enters this gas per cycle, and where does it happen? PV 0 = Constt, It is a polytropic process equation for an isobaric process. ii) Isothermal Process: In isobaric process, C=∞. If c=∞ . So polytropic process equation for isothermal process becomes: => PV 1 = Constant => PV = Constant iii) Adiabatic Process: In the Adiabatic process, C=0 . So polytropic process equation for adiabatic process ... An adiabatic thermodynamic process is an isentropic (constant entropy) process. The area of the P-V diagram in the figure below bounded by 1-2-3-4-1 is the adiabatic power. Isentropic process
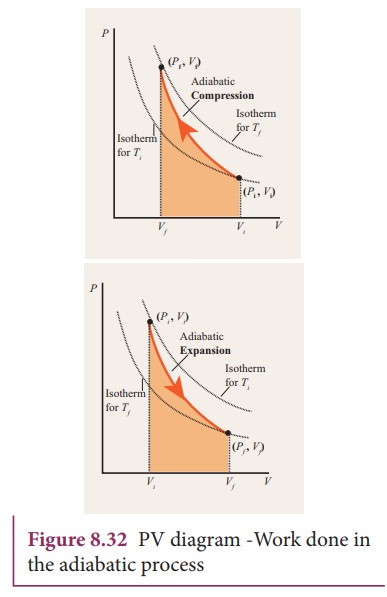
Any thermo wizards around? For the past few weeks, I have been trying to wrap my head around calculating work, temperature, and changes in internal energy during adiabatic and isobaric/thermal/choric processes. Does anyone know of any decent resources for practice problems, hopefully including PV diagrams? Any particular Physics I books that make these equations a little more intuitive? I've spent some time looking through KhanAcademy; however, he doesn't spend much time going over this stuff... The work of expansion can be depicted graphically as the area under the p-V curve depicting the expansion. Comparing examples \(\PageIndex{1}\) and \(3.1.2\), for which the initial and final volumes were the same, and the constant external pressure of the irreversible expansion was the same as the final pressure of the reversible expansion, such a graph looks as follows. BC represents constant pressure process (isobaric). AC represents constant volume process (isochoric). In P-T diagram. AB will be a vertical straight line (isothermal process). BC will be a horizontal line (constant pressure process). AC will be a positive slope inclined line (constant volume process). Ideal gas equation. PV = mRT. for AC V ... The thermodynamic process is where the movement of heat energy takes place either within an object/area or between objects/areas. Learn about the isobaric, isochoric, isothermal, and adiabatic ...
(1) A cylinder contains 3.25 mol of a diatomic gas at 20.0◦C and pressure of 1.1 atm. A scientist heats the gas at constant volume, adding 1.6 × 104 J of heat. Then, he heats it further at constant pressure to twice its original volume. a. Draw a clear and neat pV diagram of the experiment. b. Calculate the final temperature of the gas. c. Calculate the amount of work done by the gas. d. Calculate the amount of heat added to the gas while it is expanding. e. Calculate the change in interna... 1st Law for isochoric, isothermal and adiabatic process. • Temperature Three special ideal gas processes: one of, W or Q is 0. • fix volume by 0 for isobaric. Explain the differences among the simple thermodynamic processes—isobaric, isochoric, isothermal, and adiabatic. Description : Draw P-V and T-S diagram of dual combustion cycle. Last Answer : Dual combustion cycle: 1-2 Isentropic compression of air 2-3 the combustion of fuel at constant volume. 3-4 the combustion of fuel at constant pressure 4-5 Isentropic expansion during which work is done by the system. 5-1 Heat rejection at constant volume. Indicator Diagram. Adiabatic Process. Adiabatic process is a thermodynamic process in which there is no exchange of heat between the system and the surrounding from the initial to the final state. Since the heat exchange is zero \(∆Q = \rm{const} = 0\) Internal energy of the system is given by, \(∆U = nC_v ∆T\)
here , we can conclude that the slope of PV diagram of Polytropic process is negative and greater than isothermal process but less than Adiabatic process. Because , 1 < N < ¥ Therefore the P-V diagram of Polytropic process will be like :
A: cross-sectional area of a piston. dV= Adx: increase in the volume of gas. P 1, V 1, T 1: Initial state of Gas. P 2, V 2, T 2: Final state of Gas. Total Work done of the gas is given by: Also, we know that P 1 V 1 and P 2 V 2 are equal to nRT 1 and nRT 2 respectively. So the equation for work done in the adiabatic process is also given by.
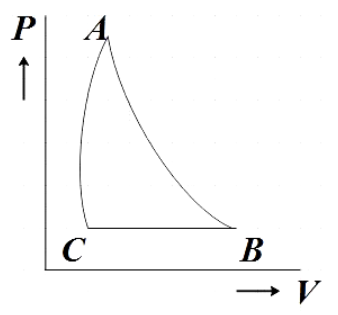
This discussion on In the P-V diagram shown. The gas does 5 J of work in isothermal process a b and 4 J in adiabatic process b c. What will be the change in internalenergy of the gas in straight path c to a?a)9 Jb)1 Jc)4 Jd)5 JCorrect answer is option 'C'.
Answer (1 of 3): It depends on how the process is carried out. If the gas is expanded reversibly against a piston, so as to produce work, then an amount of heat must be added equal to the work done to maintain the gas at the same temperature. If, instead, the gas is initially contained in part of...
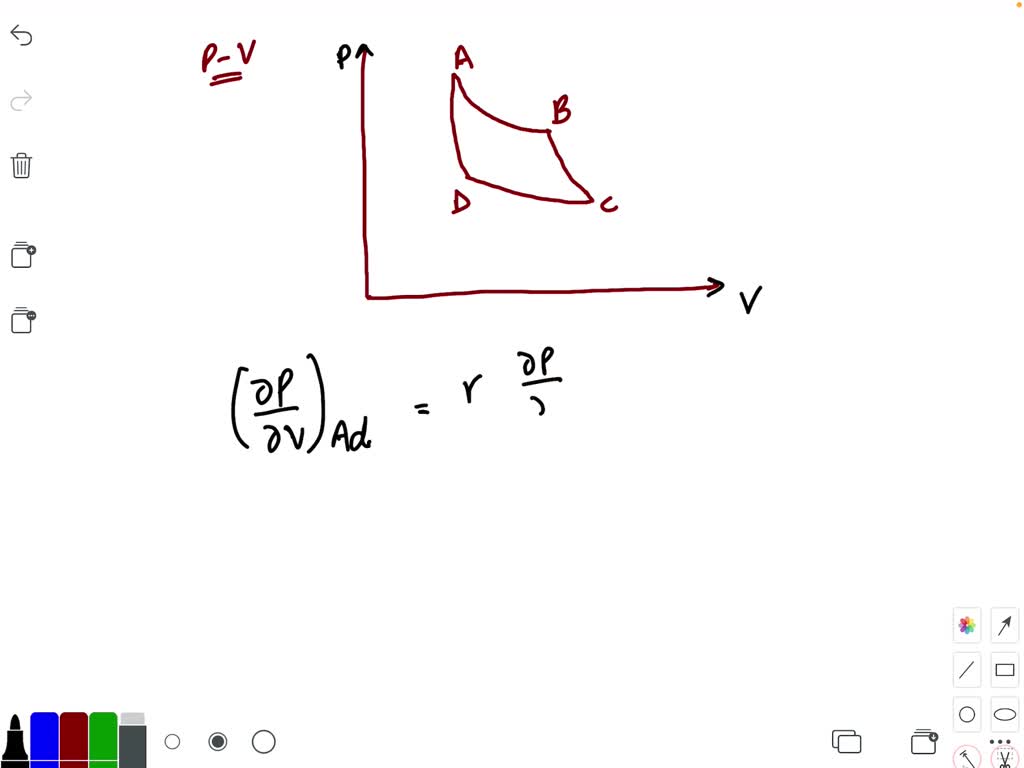
Indicator diagram (i) Curve obtained on PV graph are called adiabatic curve. (ii) Slope of adiabatic curve: From PV γ = constant. By differentiating, we get d P V γ + P γ V γ - 1 d V = 0. d P d V = - γ P V γ - 1 V γ = - γ P V. ∴ Slope of adiabatic curve tan ϕ = - γ P V (iii) But we also know that slope of isothermal curve tan θ = - P V. Hence (Slope) Adi = γ⨯(Slope ...
1st Law for isochoric, isothermal and adiabatic process. • Temperature Three special ideal gas processes: one of, W or Q is 0. • fix volume by 0 for isobaric. Explain the differences among the simple thermodynamic processes—isobaric, isochoric, isothermal, and adiabatic. Calculate total work done in a cyclical.
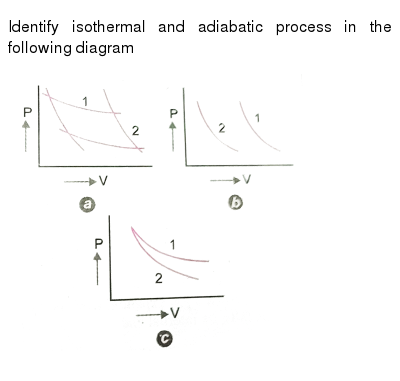
the diagrams, instead of applying PV=nRT appropriately. For example, they tended to think of a curved P-V diagram as an isothermal or adiabatic process, and in interpreting a linear P-V diagram they tended to think temperature must be constant if the pressure decreases linearly with volume.
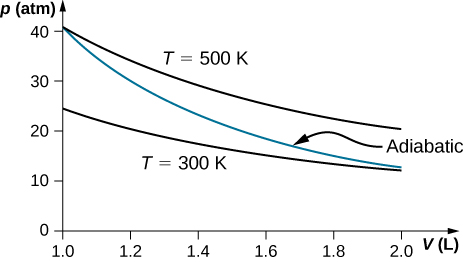
During an adiabatic process no heat is transferred to the gas, but the temperature, pressure, and volume of the gas change as shown by the dashed line. As described on the work slide, the area under a process curve on a p-V diagram is equal to the work performed by a gas during the process. On the right of the figure we have plotted the ...
In an isothermal and an adiabatic process as are taken as a reversible process. 2. Constant Volume, Constant Pressure and Constant pv n. We know that when the temperature of the hot body, supplying the heat, remains constant during the process, the temperature of the working substance will vary as the operation proceeds.
A gas undergoes a cyclic process a-b-c-a which is as shown in the PV diagram. The process a-b is isothermal, b-c is adiabatic and c-a is a straight line on P-V diagram. Work done in process ab and bc is 5 J and 4 J respectively.
77. 9. Homework Statement: A 4.00-L sample of a diatomic ideal gas with specific heat ratio 1.40, confined to a cylinder, is carried through a closed cycle. The gas is initially at 1.00 atm and 300 K. First, its pressure is tripled under constant volume. Then, it expands adiabatically to its original pressure.
First Law of Thermodynamics is a fundamental rule that relates internal energy and work done by a system to the heat supplied to it. This law has played a very significant role in some of the greatest inventions like heat engines, refrigerators, air conditioners etc. Thermodynamics is the branch of physics that deals with the study of the transfer of heat between two bodies and the resulting ...
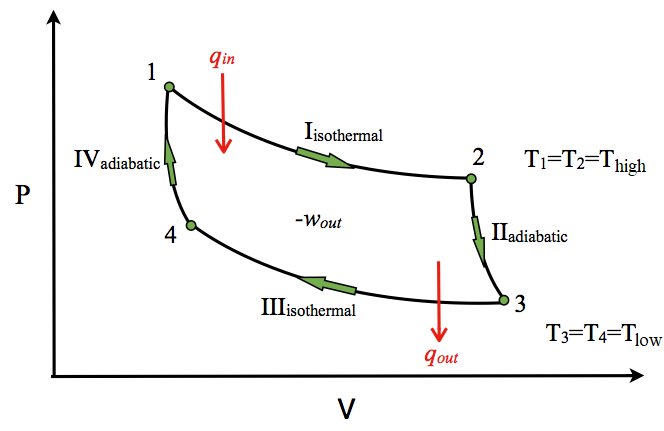
pv-&-ts-diagram-of-carnot-cycle. Here, T 1 T 1 = T 4 T 4 = T L T L &. T 2 T 2 = T L T L = T H T H. The Processes in carnot cycle are as follows:-. Reversible adiabatic compression. Reversible Isothermal expansion ( Also known as Reversible Isothermal heat addition) Reversible adiabatic expansion. Reversible isothermal compression (Also known as ...
The principle of maximum work states that for any process between two states, the work done by the system is maximised for a reversible process (and heat transfer is minimised), and that the work done by any reversible process between these two states is equal. I don't see how that can be compatible with going between 2 states with equal entropy by different paths. First by an adiabatic expansion. Second by an isovolumetric pressurisation followed by an isobaric expansion. The area below of the...
The above diagram represents a graph between temperature T and entropy S for a reversible Carnot cycle. Like the PV diagram, the processes for a TS diagram of a complete Carnot cycle is similar, such as, Line AB = Isothermal expansion, where the temperature T1 = Constant. Line BC = Adiabatic expansion, where the Entropy S2 = Constant.
1st Law for isochoric, isothermal and adiabatic process. • Temperature Three special ideal gas processes: one of, W or Q is 0. • fix volume by 0 for isobaric. Explain the differences among the simple thermodynamic processes—isobaric, isochoric, isothermal, and adiabatic. Calculate total work done in a cyclical.
The internal energy of systems reduces, resulting in a temperature drop. The relationship between Pressure and Volume in an adiabatic process with ideal gas is given by, PV = γ = Constant . where γ=Cp/Cv. If an ideal gas changes state adiabatically from (P 1, V 1) to (P 2, V 2): P 1 V 1 =P 2 V 2. PV diagram for the adiabatic is,


















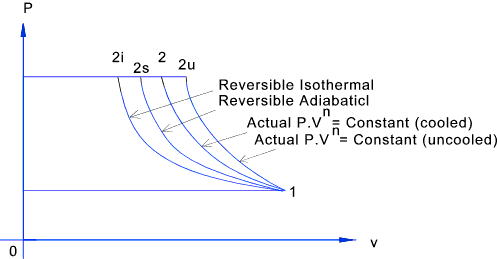

0 Response to "39 adiabatic process pv diagram"
Post a Comment