39 atom energy level diagram
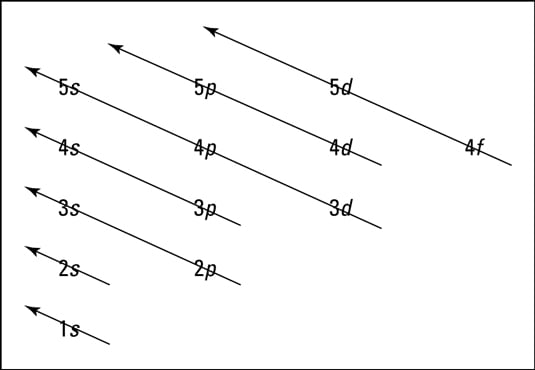
Energy Level Diagram - Different Energy Shells Around the. 9 hours ago What is energy level diagram? 2 hours ago The energy level diagram gives us a way to show what energy the electron has without having to draw an atom with a bunch of circles all the time. Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. Shown here is the first Balmer transition, in which Electrons fill in shell and subshell levels in a semiregular process, as indicated by the arrows above. After filling the first shell level (with just an s...
Figure C 1.4.8. (a) An energy level diagram showing the shift of Zeeman levels as the atom moves away from the z = 0 axis. The atom encounters a restoring force in either direction from counteriDropagating light beams, (b) A typical optical arrangement for implementation of a...
Atom energy level diagram
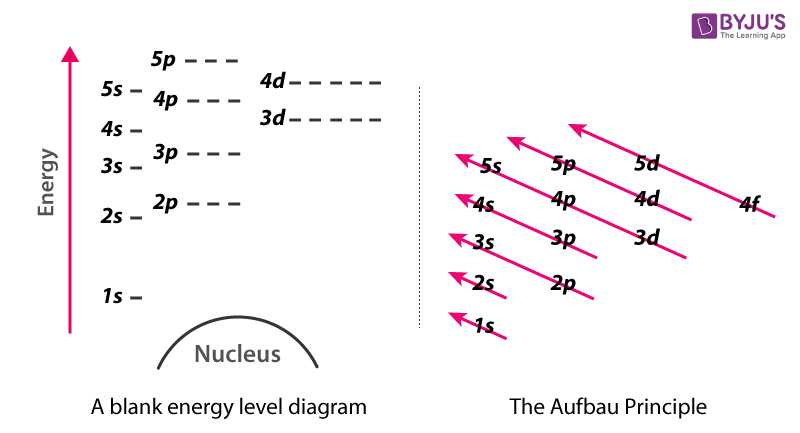
Energy level diagrams can be useful for visualizing the complex level structure of multi-electron atoms. Forms of such diagrams are called Grotrian diagrams ... We like representing these energy levels with an energy level diagram. The energy level diagram gives us a way to show what energy the electron has without having to draw an atom with a bunch of circles all the time. The energy of atomic orbitals increases as the principal quantum number, n, increases. In any atom with two or more electrons, the repulsion Figure 6.25 The diagram of an electron configuration specifies the subshell (n and l value, with letter symbol) and superscript number of electrons.
Atom energy level diagram. The Xe atom energy level diagram in Fig. 1 serves as a guide through the complex spectra (the range around Xe 6 d [3/2] 1 is omitted). ... I will teach you how to draw energy level diagrams, which is a better way of visualizing how electrons are laid out within an atom. AS Revision Questions Quantum Phenomena And Electricity AS Revision questions - Quantum Phenomena and Electricity Draw, on the diagram, an arrow between two energy levels which shows the transition responsible for the emission of a photon of Show that the electron can excite the atom... The nucleus, like the atom, has discrete energy levels whose location and properties are governed by the rules of quantum mechanics. 6-1. Energy level diagram of some of the excited states of the C12 nucleus. The angular momentum (J), parity (P), and isospin (T) quantum numbers of the states are...
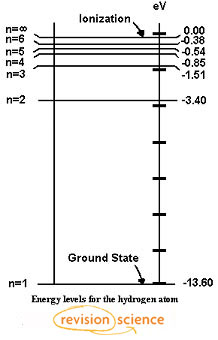
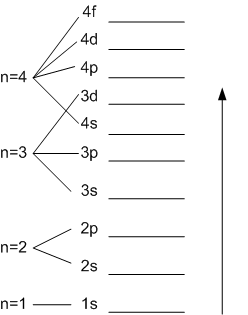
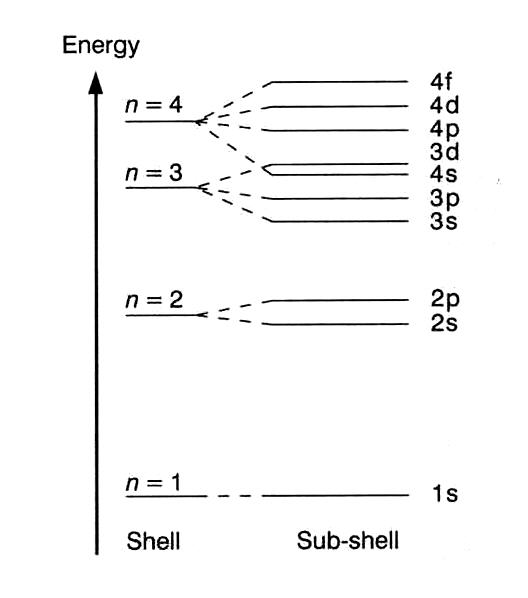
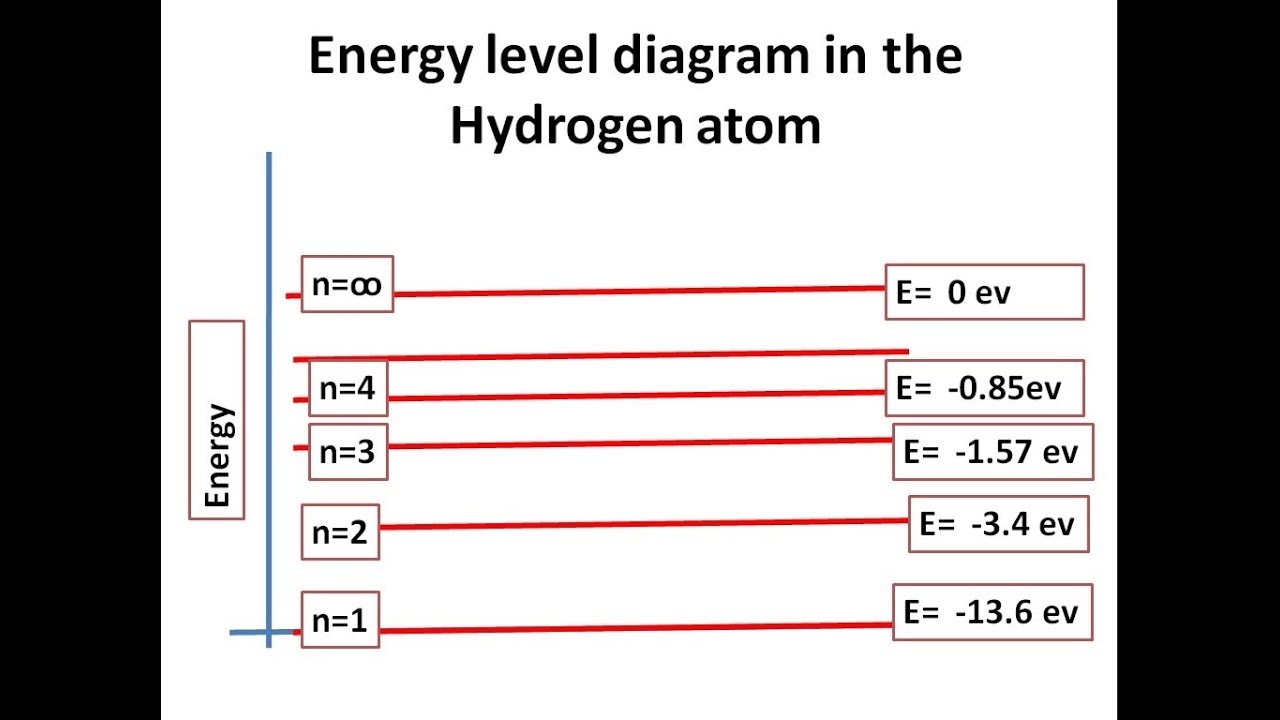
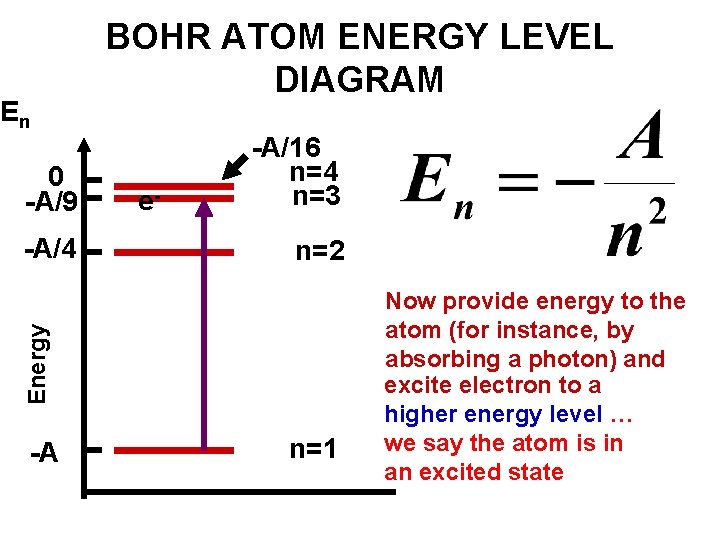
Details: Energy level diagrams and the hydrogen atom. It's often helpful to draw a diagram showing the energy levels for the particular element you're interested in. The diagram for hydrogen is shown above. The energy-level diagram for hydrogen is in fact the simplest of all atomic energy-level diagrams, just as you might have supposed. You can imagine the energy levels in a hydrogen atom as being a little like the rungs of a ladder that is sunk into a deep pit. In this analogy, the lowest rung of the ladder... From the energy level diagram in Figure 7.10, the electron energy levels go in the order 1s, 2s, 2p, 3s, 3p, 4s, 3d... Start filling the orbitals until all 16 Chlorine atoms tend to gain electrons more than sodium or calcium atoms for this same reason; Zeff for the valence electron energy level is greater... The sub-level 2p drawn in the energy level 1.5, i.e. in the diagram the vertical spacing is set to 1.5; this sub-energy level has two electrons: one with The syntax for molecules is very similar to that of the \atom command presented in the previous section. The energy sub-levels 1s, 2s and 2p become...
Chemistry Lesson 3.2Energy Level DiagramsPauli Exclusion PrincipleHund's RuleAufbau PrincipleDiagonal RulePractice problems#energyLevelDiagram #quantum... Taking these energies on a linear scale, horizontal lines are drawn which represent energy levels of the hydrogen atom (Fig). This diagram is known as energy level diagram. Sodium and mercury spectra. The sodium vapour lamp is commonly used in the laboratory as a source of monochromatic... The energy level diagram is used to represent the energy states available in each atom. When an electron is in an energy state, it emits nor absorbs radiation. An energy level diagram is more useful and easier to work with than quantum numbers in the quantum mechanical model. Chemists use the energy level diagram as well as electron configuration notation to represent which energy level, subshell, and orbital are occupied by electrons in any particular atom.
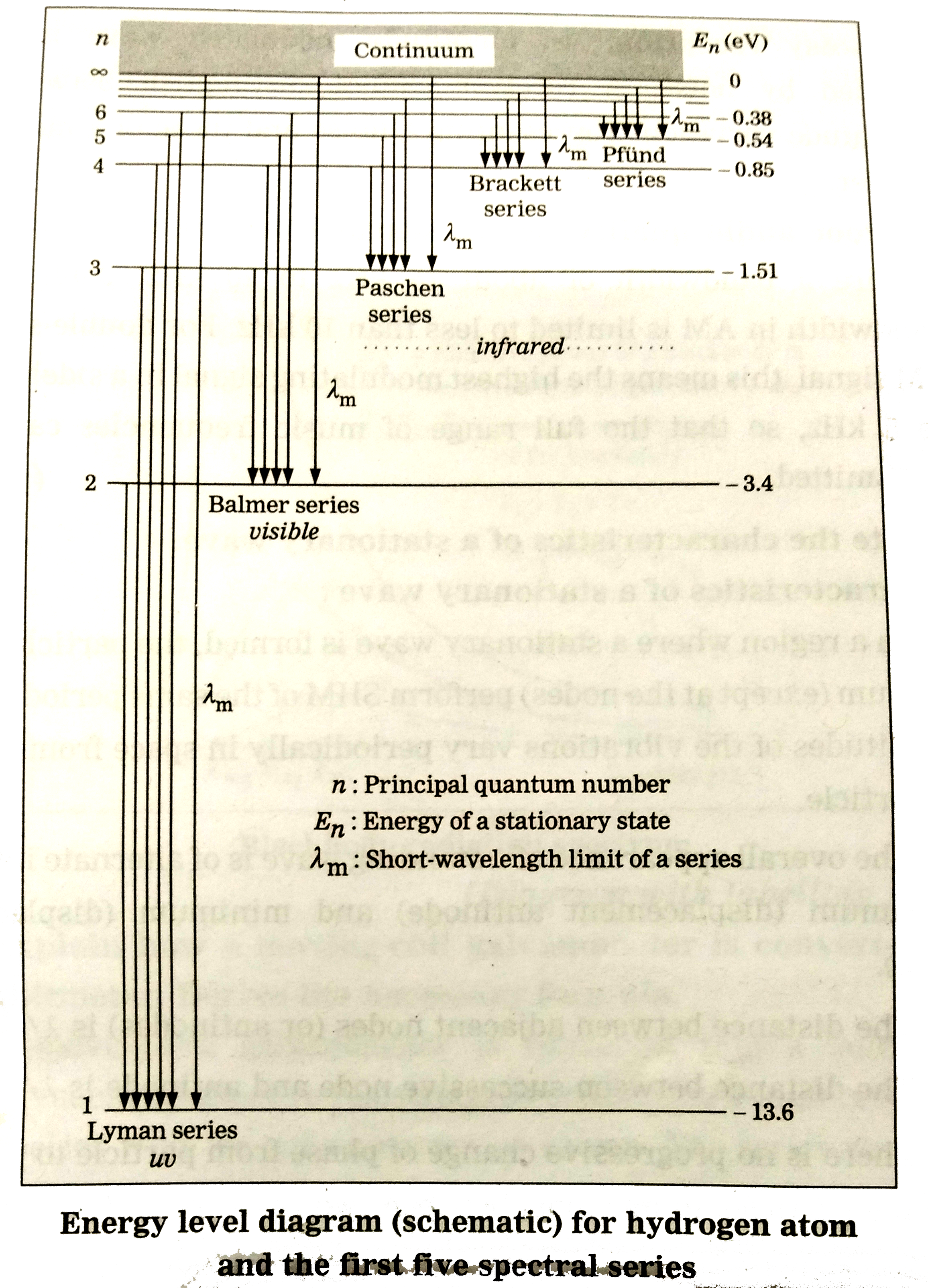
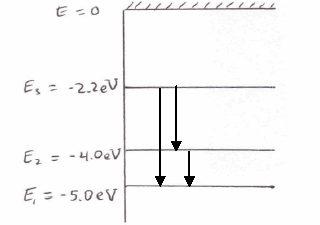
Its energy levels are given in the diagram below. The x-axis shows the allowed energy levels of electrons in a hydrogen atom, numbered from 1 to 5. The y-axis shows each level's energy in electron volts (eV).
Fig.1.1. Diagram of the energy levels and quantum transitions of the electron of a hydrogen atom. For the atomic electron, this requirement leads to the existence of three quan-tum numbers: n, ℓ, and mℓ , which define an orbital by giving the electron shell, the subshell, and the orbital within that subshell.
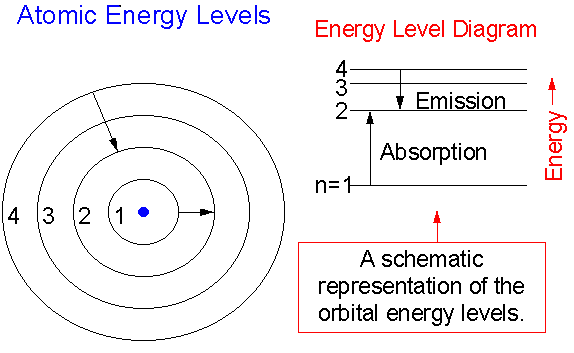
Start studying 1.4: Atomic Energy Levels. Learn vocabulary, terms and more with flashcards, games and other study tools. Ground state: lowest energy state of an atom Excited state: when an atom absorbs a photon Energy level diagram: depicts the changes in energy of an atom.
ØDraw an energy-level diagram for the orbitals in a many-electron atom and describe how electrons populate the orbitals in the ground state of an atom, using the Pauli exclusion principle and Hund's rule. ØUse the periodic table to write condensed electron configurations and determine the number of...
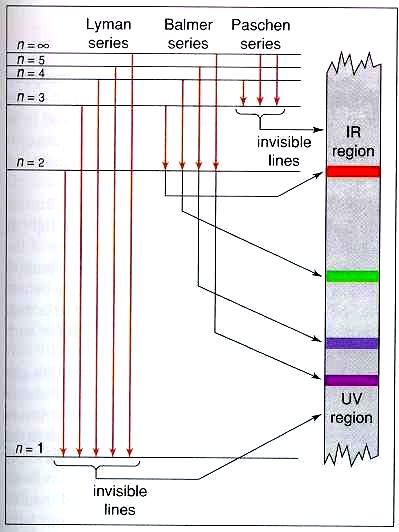
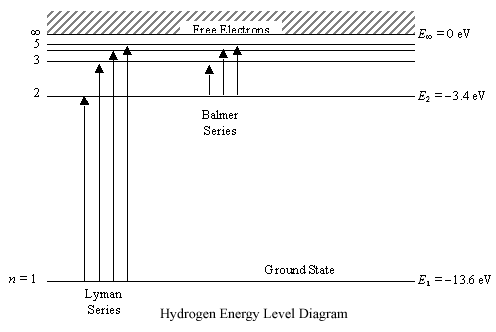
Energy-level diagrams are used for many systems, including molecules and nuclei. A theory of the atom or any other system must predict its energies based on the physics of the system. Figure 7. Energy-level diagram for hydrogen showing the Lyman, Balmer, and Paschen series of transitions.
Energy level diagram for the H atom. The origin of this formula was not understood at the time, but we now know: 4. • The lines result from electron transitions with a final energy level of n = 2. • The frequency values can be accurately calculated using the relationship E = h .
Energy level diagrams are a means of analyzing the energies electrons can accept and release as they transition from one accepted orbital to another.
Important observations from energy level diagrams of multi electron atoms are Difference of energy in d sub shell of multi electron atoms. In hydrogen atom, the only force of interaction is the force of attraction between the negatively charged electron and the positively charged nucleus.
Answer: The energy level diagram shows the existing and progressive relative energy levels between the reactants and products. Grotrian Diagram helps in understanding the allowed transition of electrons in between the atom energy levels.
It's often helpful to draw a diagram showing the energy levels for the particular element you're interested in. The diagram for hydrogen is shown above. If the electron in the atom makes a transition from a particular state to a lower state, it is losing energy.
Visualising energy levels. In an atom, electrons around a central nucleus can only have particular energy values. In the energy level diagram(below), energies have to be measured relative to one another. Remember the ground state is the lowest level and ionisation is the highest.
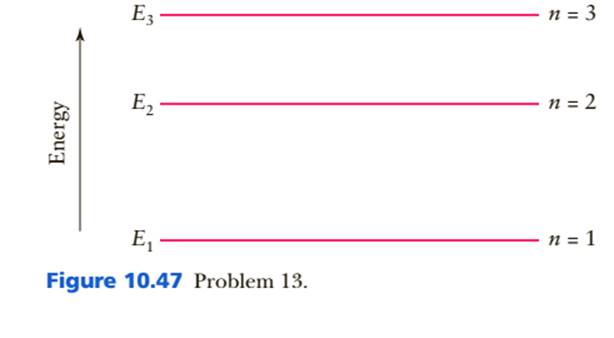
Part of a series of articles about. Quantum mechanics. Schrödinger equation. Introduction. Glossary. History. v. t. e. A quantum mechanical system or particle that is bound—that is, confined spatially—can only take on certain discrete values of energy, called energy levels.
The energy level of the electron of a hydrogen atom is given by the following formula, where. nn. n denotes the principal quantum number The figure below shows the electron energy level diagram of a hydrogen atom. Observe how the lines become closer as.
Energy levels inside an atom are the specific energies that electrons can have when energy occupying specific orbitals. An energy level diagram is exactly what it says: it is a diagram showing levels of energy. In quantum mechanics, it is a graphical representation of the available energy...
Energy levels are nothing but the fixed distances of electrons from the nucleus of an atom. Energy level diagrams are the representation of placements or arrangements of orbitals (also known as subshells) according to their increasing energy levels.
A Grotrian diagram, or term diagram, shows the allowed electronic transitions between the energy levels of atoms. They can be used for one-electron and ...
Chemistry Lesson - 12 - Energy Level Diagram and Electron Configuration. 111,476 views111K views. Oct 22, 2011.
Energy level diagrams. There are various types of energy level diagrams for bonds between atoms in a molecule.
The energy of atomic orbitals increases as the principal quantum number, n, increases. In any atom with two or more electrons, the repulsion Figure 6.25 The diagram of an electron configuration specifies the subshell (n and l value, with letter symbol) and superscript number of electrons.
We like representing these energy levels with an energy level diagram. The energy level diagram gives us a way to show what energy the electron has without having to draw an atom with a bunch of circles all the time.
Energy level diagrams can be useful for visualizing the complex level structure of multi-electron atoms. Forms of such diagrams are called Grotrian diagrams ...






















0 Response to "39 atom energy level diagram"
Post a Comment