40 ch4 molecular orbital diagram
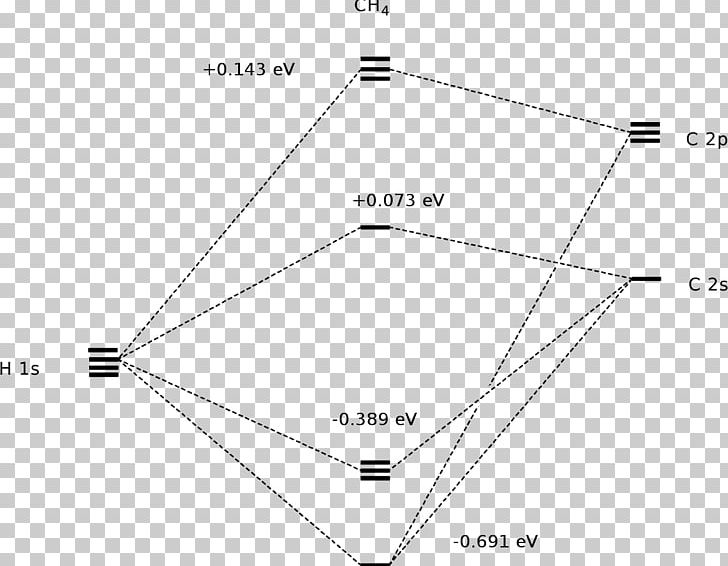
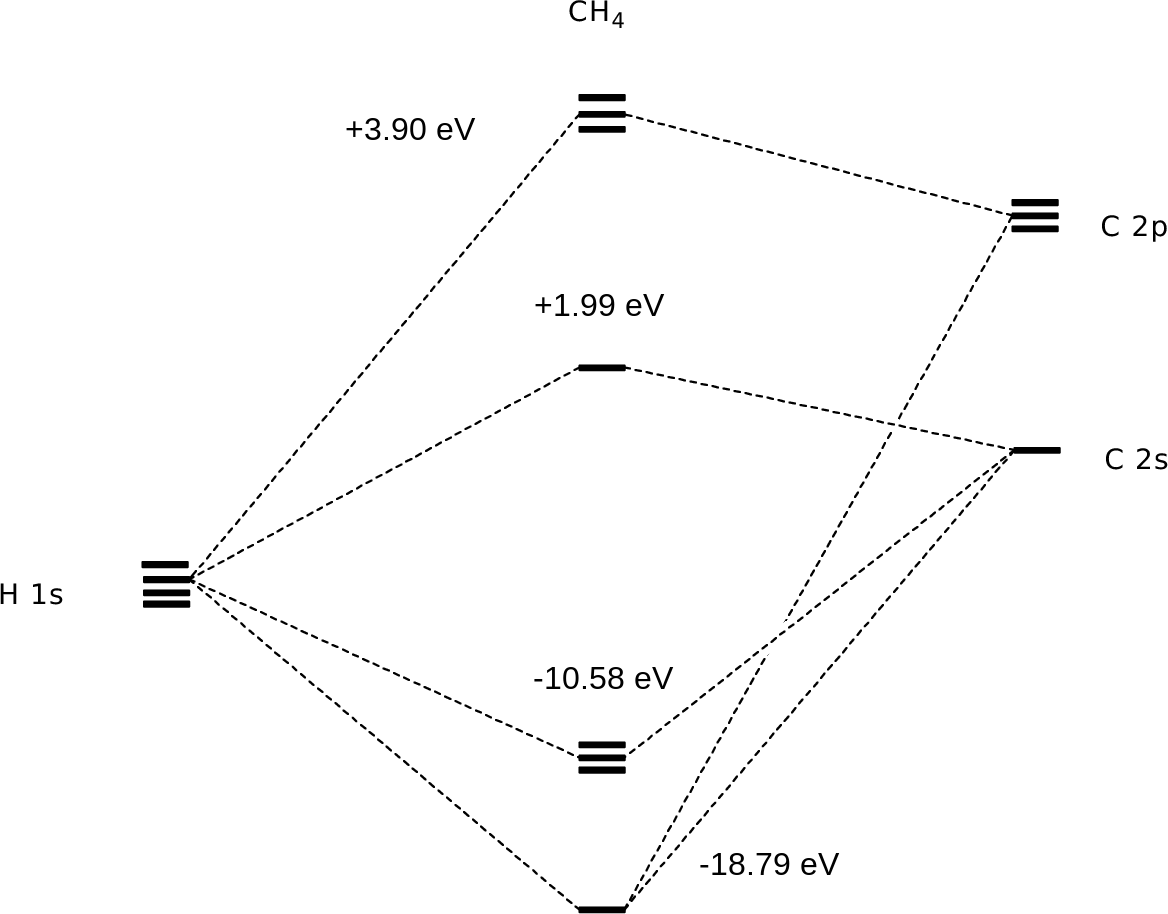
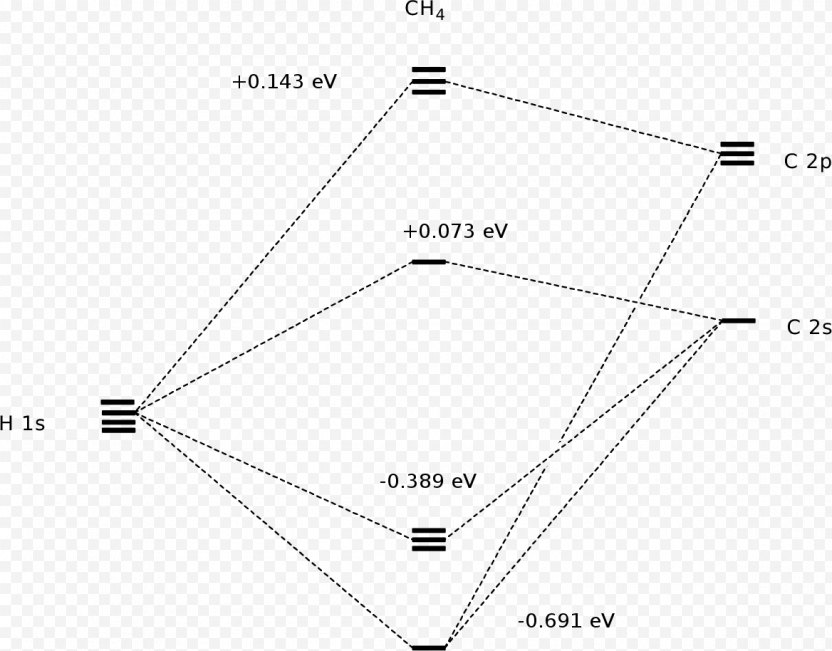
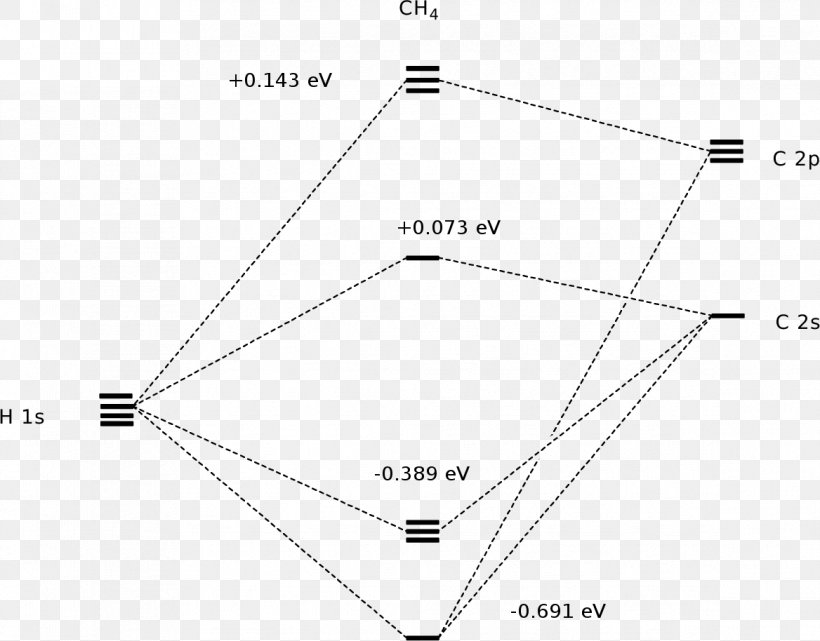
Molecular orbital diagram of ch4 the molecular orbital diagram helps with determining how mixing and overlapping have taken place in a molecule to conclude upon the hybridization type. As per the figure the four sp3 hybrid orbitals of the carbon mixes and overlaps with four 1s atomic orbitals of the hydrogen.
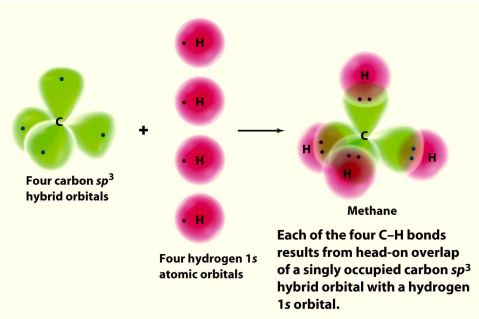
CH4 Hybridization. There are four sigma bonds between C and H. The carbon atom in the centre is sp3 hybridized. This is because, in the valence shell of carbon, one 2s orbital and three 2p orbitals combine to produce four sp3 hybrid orbitals of equal energy and shape.
Molecular orbital theory Features of Molecular orbital theory 1) The atomic orbitals overlap to form new orbitals called molecular orbitals. When two atomic orbitals overlap or combine ,they lose their identity and form new orbitals. The new orbitals thus formed are called molecular orbitals. 2) Molecular orbitals are the energy states of a molecule in […]
Ch4 molecular orbital diagram
Molecular Orbital Diagram for CH2Cl2. The premise of molecular orbital (MO) theory is that all the constituent atoms contribute towards the formation of molecular orbitals, which are a linear combination of the atomic orbitals. As per this theory, the electrons in a molecule are not individually assigned to atomic orbitals but to molecular ...
Molecular Orbital diagram of CH4 The molecular orbital diagram helps with determining how mixing and overlapping have taken place in a molecule to conclude upon the hybridization type. As per the figure, the four sp3 hybrid orbitals of the carbon mixes and overlaps with four 1s atomic orbitals of the hydrogen.
Answer: Yes, MOT is compatible for all models that correctly describe the shape of a molecule, because MOT is the perfect description of bonding, it is never wrong. The problem is that MOT is cannot give you an easy qualitative prediction of the shape of molecules, because finding the Molecular o...
Ch4 molecular orbital diagram.
The molecular orbital diagram of CO2 is as below. A molecular orbital diagram of any compound gives us an idea about the bonding of the orbitals. It also helps us to find the bond order, bond length, bond strength of the molecule. In the diagram, the left-hand side consists of the atomic orbitals of carbon.
Molecular Orbital diagram of CH4 The molecular orbital diagram helps with determining how mixing and overlapping have taken place in a molecule to conclude upon the hybridization type. As per the figure, the four sp3 hybrid orbitals of the carbon mixes and overlaps with four 1s atomic orbitals of the hydrogen.
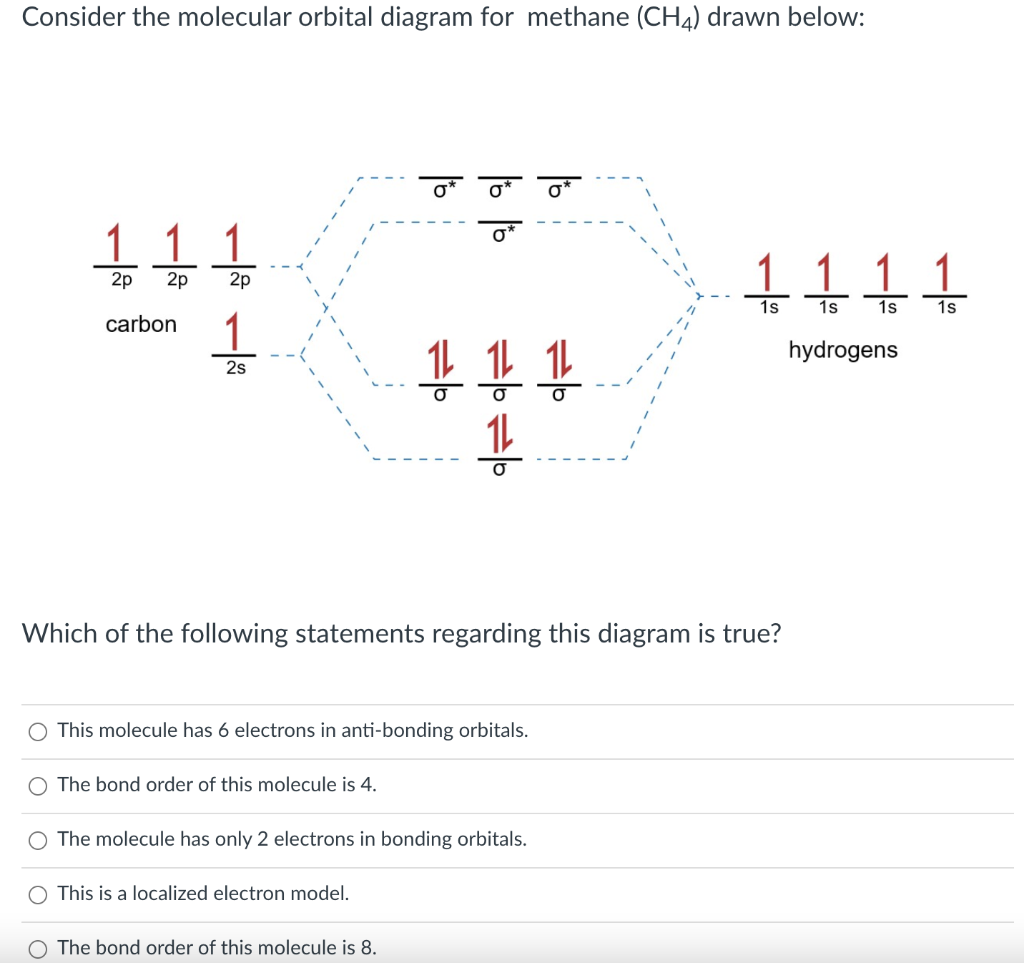
Bond Order. Bond order is defined as half of the difference between the number of electrons present in bonding and antibonding molecular orbitals. The bond order may be a whole number, a fraction or even zero. It may also be positive or negative. Bond order (B.O.) = 1 2[N b − N a] 1 2 [ N b - N a] (vi).
The problem is that MOT is cannot give you an easy qualitative prediction of the shape of molecules, because finding the Molecular orbitals requires complex ...1 answer · 1 vote: Yes, MOT is compatible for all models that correctly describe the shape of a molecule, because ...
The rules to fill molecular orbitals are the same, except that each "bonding" orbital is followed by an "anti-bonding" orbital. While atomic orbitals are filled as 1s2s2p… molecular orbitals are filled as 1s1s*2s2s*2p…. The asterisked orbitals represent anti-bonding orbitals.
The approach used might be utilized to any mole to gram conversion. 3 moles CH4 to grams 4812738 grams. What Are Hybrid Orbitals Grasp Natural Chemistry Natural Chemistry Chemistry Molecular Geometry - calculate mass of magnesium steel. What number of moles are in 8 grams of ch4. 2 moles CH4 to grams 3208492 grams. What's
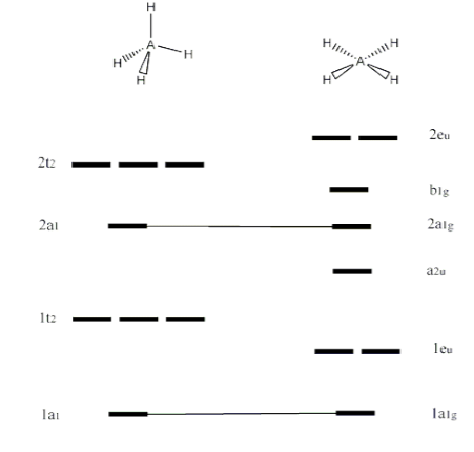
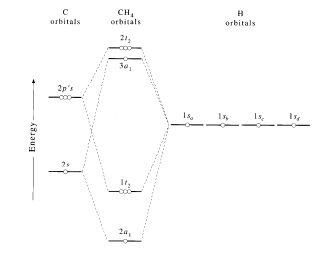
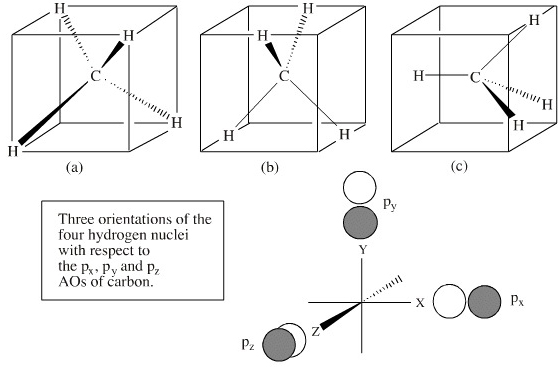
Introduction Various concepts explain the molecular orbital theory in the bonding in methane, including character tables, symmetry, LGOs (ligand group orbital approach), and a qualitative MO diagram. The Symmetry of CH 4 CH 4 belongs to the T d point group and contains: 8C 3 axes, 3C 2 axes, 6S 4 axes, and a dihedral plane of symmetry.
Molecular Orbital diagram of CH4 The molecular orbital diagram helps with determining how mixing and overlapping have taken place in a molecule to conclude upon the hybridization type. As per the figure, the four sp3 hybrid orbitals of the carbon mixes and overlaps with four 1s atomic orbitals of the hydrogen.
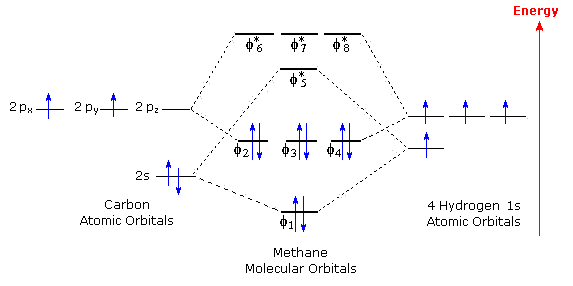
Properties and bonding. Methane is a tetrahedral molecule with four equivalent C-H bonds.Its electronic structure is described by four bonding molecular orbitals (MOs) resulting from the overlap of the valence orbitals on C and H.The lowest-energy MO is the result of the overlap of the 2s orbital on carbon with the in-phase combination of the 1s orbitals on the four hydrogen atoms.
C2H4 Molecular Orbital (MO) Diagram The molecular orbital theory is a concept of quantum mechanics where atomic linearly combines to form molecular orbitals and we describe the wave nature of atomic particles. Here, bond strength depends on the overlapping degree which in turn depends on the spatial proximity of the combining atoms.
Aug 15, 2020 — Most general chemistry textbooks invoke sp3 hybridization to explain the bonding in the tetrahedral methane (CH4) molecule. The idea (valence ...
The above-mentioned molecular orbital diagram of acetylene (C2H2) is specifically showing the Carbon-Carbon bond. You can see how the sp hybridized orbitals combine and overlap to form a bonding sigma (σ) orbital and an antibonding sigma (σ*) orbital.
MO diagram of homonuclear diatomic molecules ... 4) MO theory and molecular geometry (Walsh diagrams) ... Molecular Orbital Theory – LGOs for methane.29 pages
Hybridization is vital to understand the molecular geometry of the compound. When the two or more orbitals hybridize, the orbital is known as the hybrid orbitals. These orbitals are formed when there are bond formations in the compound. For this compound, there are four covalent bonds between the central Carbon atom and four chlorine atoms.
Jun 22, 2020 — Molecular Orbitals: Methane ... The molecular orbital description of bonding in methane does several things for us. ... The bottom 4 are all ...
b) A molecular orbital is singly occupied. c) An example is oxygen molecule. d) Repelled by the magnetic field. Answer: Repelled by the magnetic field. 48. Combination of two atomic orbitals results in the formation of two molecular orbitals namely. a) one bonding and one non-bonding orbital. b) two bonding orbitals. c) two non-bonding orbitals
Molecular orbit diagram of CH4 The molecular orbit diagram helps with determining exactly how mixing and overlapping have taken location in a molecule come conclude upon the hybridization type. As every the figure, the four sp3 hybrid orbitals of the carbon mixes and overlaps with 4 1s atom orbitals of the hydrogen.
The Lewis structure of the methane (CH4) molecule is drawn with four single shared covalent bonds between the carbon and hydrogen atoms each. Moreover, as there exist sigma bonds only and one 2s and three 2p orbitals of the carbon produce four new hybrid orbitals, the hybridization of CH4 is sp3.
SF4 Molecular Geometry. ... Hence the sulfur atom uses five hybridized orbitals, one 3s orbital, three 3p orbitals, and one 3d orbital. This arrangement of electrons around the atom and hybridized orbitals leads to the sp3d hybridization. One can also use the steric number to know the hybridization; here, the steric number is 5 for the sulfur atom.
The molecular geometry of a compound is the 3-D representation of the atoms in the molecule. It also depicts the shape and bonds in the molecule. ... In its electron configuration, the C atom has 2 electrons in s and 1 electron each in 2px and 2pz orbitals.
Molecular orbitals like atomic orbitals obey Aufbau principle for filling of electrons. Answer: C ( Bonding molecular orbital has higher energy than antibonding molecular orbital. Question 26: The conditions for the combination of atomic orbitals to form molecular orbitals are stated below.
It is possible to see how complex the orbital structure becomes with the increase in energy. Methane has four valence molecular orbitals (bonding), consisting ...
CH4 Bond Angles One can use AXN Notation to find out the molecular geometry and the bond angles for any molecule. Here CH4 follows the AX4 notation, and hence according to the table given below, the bond angles are 109.5° The CH4 molecule will have 109.5° bond angles as there is no distortion in its shape.
A molecule is paramagnetic when it contains half-filled molecular orbitals. Question 47. Arrange the following molecular species in increasing order of stability. Answer: N 2 2-< N 2-= N 2+ < N 2. Question 48. Explain on the basis of the molecular orbital diagram why O 2 should be paramagnetic? Answer:
A molecular orbital diagram of any type of compound provides us an idea about the bonding that the orbitals. It likewise helps united state to uncover the link order, link length, bond stamin of the molecule. In the diagram, the left-hand side is composed of the atom orbitals the carbon. Likewise, the left side has actually AO's the oxygen.
Draw a molecular orbital diagram for (a) H2, (b) H2 O, (c) BHs, (d) CH4, and (e) [Fe (H)ol by performing the following steps i. Determine the point group of the molecule. Do not descend in symmetry ii. Determine the reducible representation (THIs) using the hydrogen 1s orbital s as your basis set. 111, Express the reducible representation as a ...
In the case of methane, this model implies four eqivalent localized orbitals between the hydrogen atoms and the central carbon. However, the following orbital ...















0 Response to "40 ch4 molecular orbital diagram"
Post a Comment