37 energy level diagram chemistry
Chemistry; History; Other; Contact; Posted on 1 September 2021 by lets tokmak. If applied energy to the energy level diagrams resulted in the excitation of electrons, in your own words describe a. If applied energy to the energy level diagrams resulted in the excitation of electrons, in your own words describe a phenomenon that could occur. ... When we make the molecular orbital energy level diagram of f2 molecule then, we will get this configuration: 1σs 2, 1σ*s 2, 2σs 2, 2σ* 2, σ2pz 2, π2p x2, π2p y2, πp x * 2, π2p y * 2. From this electronic configuration, we can see that there are a total of ten bonding molecular orbitals and eight antibonding molecular orbitals.
An electron in a low energy level can absorb energy and undergo a transition to a higher energy level. When that electron returns to the ground state, it loses energy by emitting a photon, which is a tiny particle that behaves like a wave and travels at the speed of light.
Energy level diagram chemistry
“You are responsible for the energy that you create for yourself, and you’re responsible for the energy th “You are responsible for the energy that you create for yourself, and you’re responsible for the energy that you bring to others.” – ... Systems with a greater number of electrons will occupy a greater amount of energy levels. The electron configuration for Lithium is: 1s 2 2s 1 . Lithium, containing three electrons, has two electrons occupying an s orbital at the first energy level, and one electron occupying an s orbital at the second energy level. UV-Visible Spectroscopy - Principle - Types of electronic transition Energy level diagram of ethane, butadiene, benzene and hexatriene. Instrumentation of UV-Visible spectrometer and applications.IR-Spectroscopy - Principle - Number of vibrational modes - Vibrational energy states of a diatomic molecule and -Determination of force constant ...
Energy level diagram chemistry. I hold a Ph.D. in Physical Chemistry, from Calgary, Canada. I have been helping students with their homework for the past 6 years. I solve assignments related to Physical Chemistry. Visit eduassignmenthelp.com or email info@eduassignmenthelp.com. You can also call on +1 678 648 4277 for any assistance with Physical Chemistry Assignments. The molecular orbital energy-level diagram, which is a diagram that shows the relative energies of molecular orbitals, for the H2 molecule is shown in Figure 13 ... Electron configuration of carbon (C) atom through orbital diagram Atomic energy levels are subdivided into sub-energy levels. These sub-energy levels are called orbital. The sub energy levels are expressed by 'l'. The value of 'l' is from 0 to (n - 1). The sub-energy levels are known as s, p, d, f. Pairing up catalysts to maximize the rate of chemical conversion In this diagram, the two "hidden" half-reactions responsible for the observed catalysis are depicted on opposite sides of a box in which the voltage level of the catalyst composite (the catalysts plus the carbon substrate) is indicated as pink. The conversion of reactant to product is on the left, and the conversion of oxygen ...
Describe the energy levels in the quantum mechanical model of the atom. How does the spacing change as the energy levels get further from the nucleus? The schematic energy level diagram of the devices containing different HTLs is shown in Figure 4 a, and the detailed preparation process is described in the see the Supporting Information. Figure S12 and Figure 4 b represent the SEM of a cross-sectional view of PSC devices with spiro-OMeTAD and V1366 , providing a direct view of the PSCs ... Energy level diagrams in various crystal fields, CFSE, applications of CFT, Jahn- Teller distortion. Electronic spectra of transition metal complexes: spectroscopic term symbols, selection rules, Orgel and Tanabe-Sugano diagrams, nephelauxetic effect and Racah parameter, charge-transfer spectra. Magnetic properties of transition metal complexes. Chemistry Lesson 3.2 Energy Level Diagrams Pauli Exclusion Principle Hund's Rule Aufbau Principle Diagonal Rule Practice problems ...
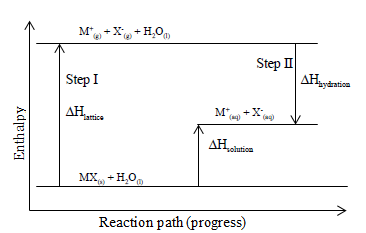
Draw an energy level diagram for the neutralization reaction in 4(c) above. Heat of Solution/Enthalpy of Solution. Is the heat change when a given mass (moles) of a substance is dissolved in a stated amount of solvent (water). Energy level diagrams. are used to model energy. changes during reactions. They show the relative energy levels of the products. and reactants . 23.07.2021 ... Chemists sometimes use an energy level diagram to represent electrons when they're looking at chemical reactions and bonding. I am asked to draw an energy level diagram that shows the relationship between the energy quantities involved in the problem. What I did was I calculated the standard enthalpy change of the reaction first (-43.16 kJ/mol-rxn) and then drew the diagram below. Did I draw the diagram correctly? [Energy Diagram I drew](https://preview.redd.it/yl8e8l4byg081.jpg?width=1280&format=pjpg&auto=webp&s=2f24a33862f2e8c7dace0351ce643c3a2666e2ac) [The Question](https://preview.redd.it/fry8myexxg0...
I’m SO CONFUSED about where/how to start drawing the energy level diagram especially when i’m given A TON of information, both on the question and in the data booklet. usually I identify the “0” point where the elements are in their respective states, then i try to fit in from there. i know WHAT i want to find, but i don’t know how to trace the steps (eg do I use the atomisation value first??? or the EA / IE value?) TL;DR: so many values given to draw energy level diagram. which do i use fi...
It seems that I can't wrap my head around the concept of the k-vector and crystal momentum. From my waves course I remember that the wave vector sorta represents where the wave propagates (in an isotropic and homogeneous material). So in solid state that could be thought as the same thing applied to the wave function, right? And here comes the troubles: why and how is it related to the momentum of a particle? I think I get the math behind it but not the concept. Furthermore, given th...
In a world that gets more hectic each day, people are always feeling run down and are looking for ways to boost their energy levels. However, many people want to avoid synthetic energy pills and drinks with powerful stimulants and have turn...
[https://www.stuvia.com/doc/982074/gizmos.-chemistry-101-student-exploration-periodic-trends-periodic-trends-gizmo.-atomic-radius-electron-affinity-electron-cloud-energy-level-group-ion-ionization-energy-metal-nonmetal-nucleus-period-periodic-trends-picometer-valence-electr](https://www.stuvia.com/doc/982074/gizmos.-chemistry-101-student-exploration-periodic-trends-periodic-trends-gizmo.-atomic-radius-electron-affinity-electron-cloud-energy-level-group-ion-ionization-energy-metal-nonmetal-nucleu...
Chemistry Lesson - 12 - Energy Level Diagram and Electron Configuration. 112,069 views112K views. Oct 22, 2011.
09.06.2020 ... Typically, at low energies, the levels are far apart. At higher energies, they are closer together. At sufficiently high energy, an electron is ...
It is defined as the amount of energy required to break one mole of bonds of a particular type between two atoms in a gaseous state. The unit of bond enthalpy is kJ mol-1. For example, the H - H bond enthalpy in hydrogen molecule is 435.8 kJ mol-1. Molecular Orbital Theory (MOT)
In chemistry, the principal energy level of an electron refers to the shell or orbital in which the electron is located relative to the atom's nucleus. STEVE HORRELL / SPL / Getty Images In chemistry, the principal energy level of an electr...
Energy level labels are different from the atomic energy levels' corresponding symbols. With the increase in quantum number l and orbital angular momentum, the energy level increases just like in the case of atomic energy levels. Here, the quantum number I is represented in terms of l = 0,1,2,3, 4, depending upon the corresponding energy level.
An energy level diagram is a great way to compare these two contrasting Systems. Energy level diagram for an Endothermic change. Thermal energy from the Surroundings is absorbed by the System. This...
Since the bond order of Li 2 is higher than Li 2 + and Li 2 - . Therefore ,Li2 has stronger single covalent bonding . Thus lithium molecule (Li2) is more stable. Li 2 + is more stable as compared to Li 2 - :. Li 2 + and Li 2 - ions have the same bond order ( 0.5) .. But Li 2 - has more electrons in higher energy antibonding molecular orbital as compared to Li 2 + .
I'm working on a lab on the decomposition of hydrogen peroxide (with MnO2) right now where the instructions say: "Draw a Ep vs RC diagram for the decomposition of hydrogen peroxide. Include energy values in the y-axis. Be sure to draw a dotted line to represent the catalyzed reaction." ​ I know what the energy diagram should look like, but what does "RC" mean? Does it just mean "Reaction Progress"? ​ Edit: Apparently RC means "reaction complex", but I don't understand ...
ZnO as an interlayer plays an important role in organic solar cells (OSCs). There are two kinds of ZnO interlayers, including sol-gel method-obtained interlayers and those obtained using ZnO nanocrystals. While sol-gel ZnO interlayers require thermal treatment, ZnO nanocrystals can generate thin film interlayers without extra treatment. However, ZnO nanocrystals in solution are usually ...
Energy Diagrams. Exothermic Reactions. Endothermic Reactions. Example. 6.3 Kinetic Energy, Heat Transfer, and Thermal Equilibrium. 6.4 Heat Capacity and Coffee-Cup Calorimetry. 6.5 Phase Changes and Energy. 6.6 Introduction to Enthalpy of Reaction. 6.7 Bond Enthalpy and Bond Dissociation Energy.
The energy spectrum of a system with such discrete energy levels is said to be quantized. In chemistry and atomic physics, an electron shell, or principal ...
The first orbital, as always, has to be 1s. Since the graph goes up to 2, there are 2 electrons in the 1s orbital. The next orbital has to be 2s and the PES indicates there are 2 electrons in this orbital. So far, the electron configuration seems to be 1s^2 2s^2. There is one more peak though, which corresponds to the 2p orbital.
Hello Debra,In an exothermic reaction the products are more energetically stable than the reactants so are lower on the potential energy level diagram, i.e. potential energy is converted into kinetic energy during the reaction and heat is evolved...
The electrons revolve in their orbits without losing energy. An electron moves from lower to higher states by gaining the energy and moves from higher energy states to lower energy states by losing energy. The lowest energy level of an electron is called the Ground state. The energy levels are represented by an integer known as the Quantum number.
In chemistry, an electron shell, or energy level, may be imagined as an orbit with electrons around the nucleus of an atom. The closest shell to the nucleus is ...
An electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. Electrons are represented by dots or crosses and are positioned in energy levels, or 'shells', around the central nucleus. This is sometimes called the Bohr, or the 'solar system', model. Download this
For example, the energy of 2p orbital (l = 1) is higher than that of the 2s (l = 0) orbital, general, energies of the orbitals belonging to the same main energy level follow the order s < p < d < f Energy level diagram for the few electronic shells of a multi-electron atom (c) When n > 3, the same orbitals belonging to a lower main energy level ...
omg what. How. Help.
This distance learning lesson covers the content for the Year 8 AQA Activate 6b 6.4.2: energy level diagrams lesson from the 6b: Reactions chapter. Students work through the digital worksheet completing a variety of tasks to develop their understanding of how to use energy level diagrams to represent exothermic and endothermic reactions.
03.05.2020 ... Diagram representing the arrangement of orbitals in order of their increasing energies are called energy level diagrams.
5s: 1 5p: 3 5d: 5 5f: 7 5g: 9 1+3+5+7+9=25 she says that it's 16 cause there is no g in the periodic table. But still, she also admitted that "theoretically", g exists. High school science courses mainly revolve around numerous "theories" whatever the subject is, whether if it is biology physics env sci, or chemistry. Idk why this should be marked wrong. I checked with google and it clearly says it's 25 as well.
Energy Level Diagrams · An energy level diagram is a diagram that shows the energies of the reactants, the transition state(s) and the products of the reaction ...
The change in energy for the above reaction is represented in the energy level diagram given below. SN2 is a single-step reaction, so the diagram only shows one curve. The lower energy of the products CH 3 OH and Br - compared to the reactants CH 3 Br and OH - suggests that the reaction is exothermic and that the products are more stable.
Dear community, could someone explain to me, why Xe only puts 1 p-orbital into the bond? Where are the other 2? [This picture](https://i.stack.imgur.com/4Wuhw.jpg) shows really well, what I mean. In the above case of NO, the N puts in 3 p-orbitals with 1 electron each and O puts in 3 with 2-1-1. This leads to 6 MOs, 3 of whch are bonding and 3 of which are anti-bonding. Now beneath, it shows XeF2. Shouldn't both F bring in 3 p-Orbitals each with 2-2-1 and Xe 3 p-orbitals with 2-2-2? And...
Draw two electrons in the first energy level and label them with their charge. Some of the worksheets for this concept are atomic structure work name section parts of the atom chemistry of matter what is matter an atom apart km 654e 20150109102424 drawing atoms work.
The energy-level diagram outlines the mechanism: After excitation from the ground state (purple arrow), the molecule makes a brief stop on the black manifold of e3 Π g states, as predicted by Herzberg, before relaxing to the red manifold of d3 Π g states. It's on those d3 states that the two carbon atoms dissociate (red dashed horizontal arrow).
Endothermic Energy Profile - 9 images - bond enthalpy definition energy change solved examples, ocr chemistry unit 2 module 3 enthalpy profile diagrams,
11.11.2021 ... Energy level diagram is a part of CBSE Class 11 chemistry first term syllabus. · It carries a total of 12 periods and 5 to 6 marks. · Atomic ...
The energy levels are tracked using an enthalpy diagram. An enthalpy diagram plots information about a chemical reaction such as the starting energy level, how much energy needs to be added to...
UV-Visible Spectroscopy - Principle - Types of electronic transition Energy level diagram of ethane, butadiene, benzene and hexatriene. Instrumentation of UV-Visible spectrometer and applications.IR-Spectroscopy - Principle - Number of vibrational modes - Vibrational energy states of a diatomic molecule and -Determination of force constant ...
Systems with a greater number of electrons will occupy a greater amount of energy levels. The electron configuration for Lithium is: 1s 2 2s 1 . Lithium, containing three electrons, has two electrons occupying an s orbital at the first energy level, and one electron occupying an s orbital at the second energy level.
“You are responsible for the energy that you create for yourself, and you’re responsible for the energy th “You are responsible for the energy that you create for yourself, and you’re responsible for the energy that you bring to others.” – ...

0 Response to "37 energy level diagram chemistry"
Post a Comment