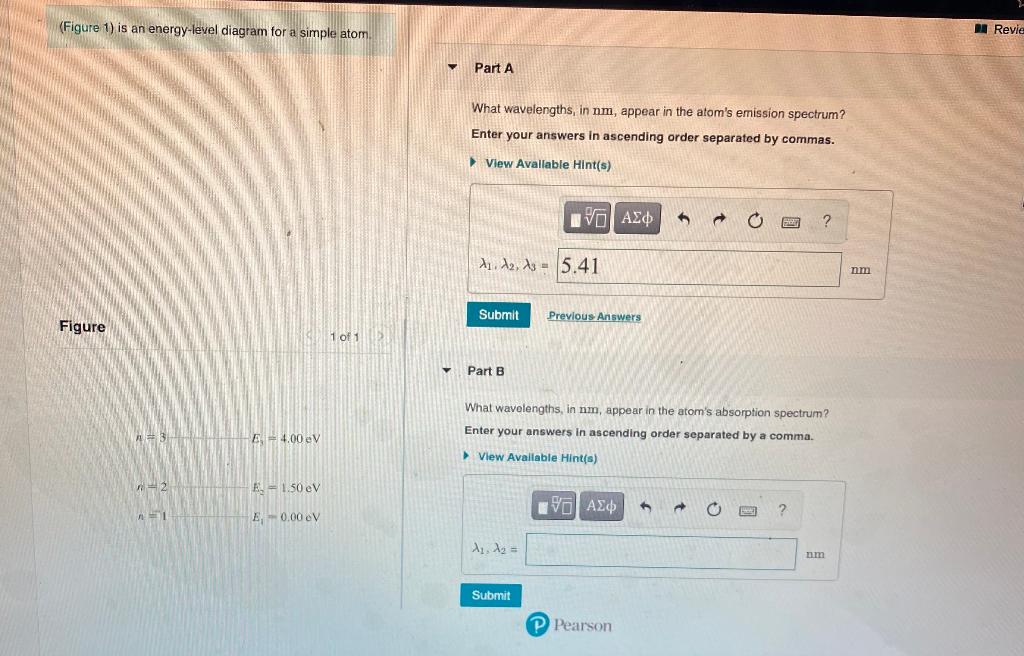
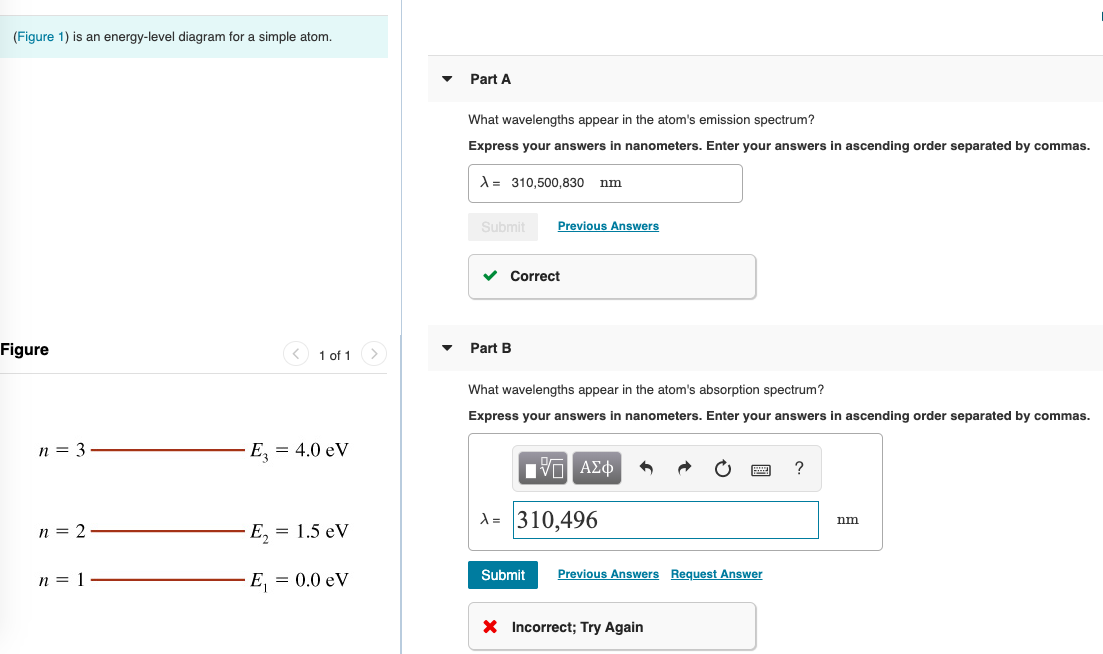
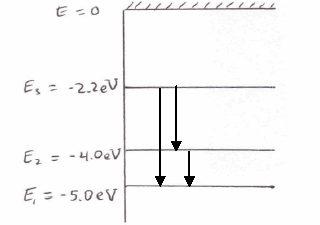
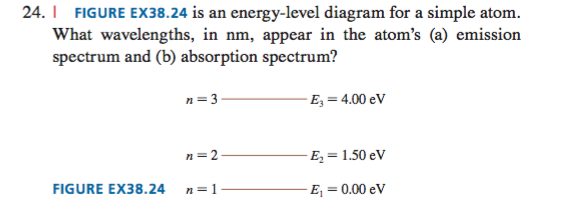
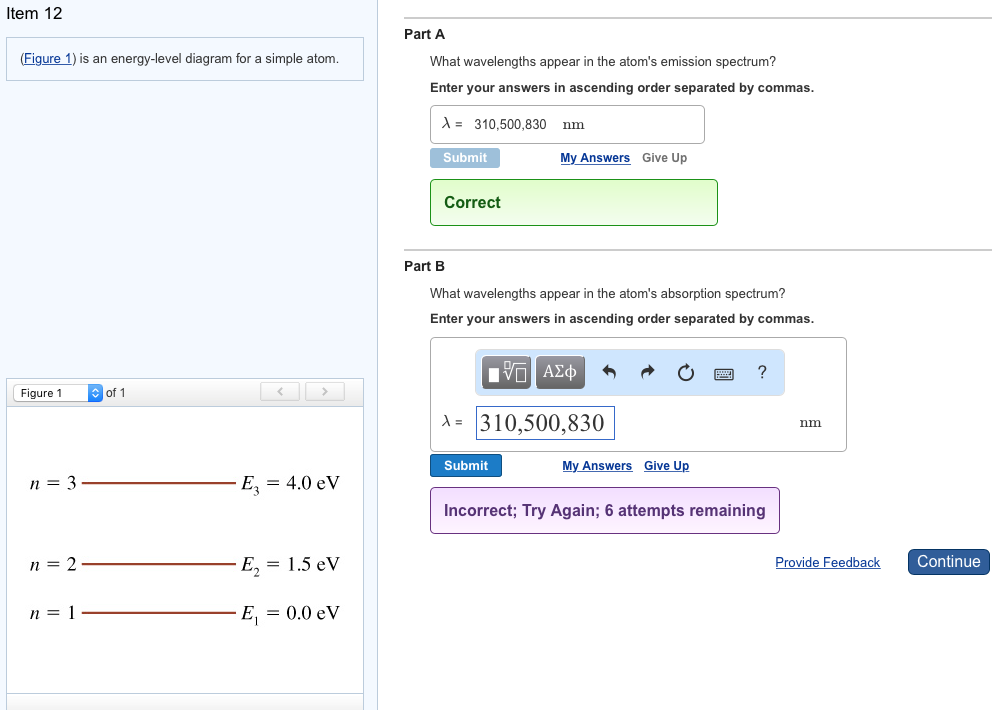
37 the figure is an energy-level diagram for a simple atom. (figure 1)
And so in the first energy level, you can fit a maximum of two electrons. And so this is going to important when we're drawing our dot structures. Because when we're drawing hydrogen, we're always going to surround it by two electrons, or a single covalent bond. When you take the second period on the periodic table, so here we are on the second period, lithium has one … In above shown figure, the energy levels of a hypothetical atom are shown. Calculate the energy of photon emitted when a electron is making transition from the third energy level to the second energy level.
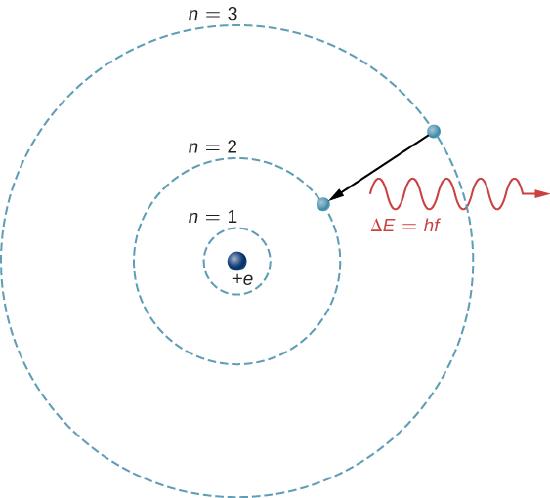
Figure 5. An energy-level diagram plots energy vertically and is useful in visualizing the energy states of a system and the transitions between them. This diagram is for the hydrogen-atom electrons, showing a transition between two orbits having energies E4 and E2.
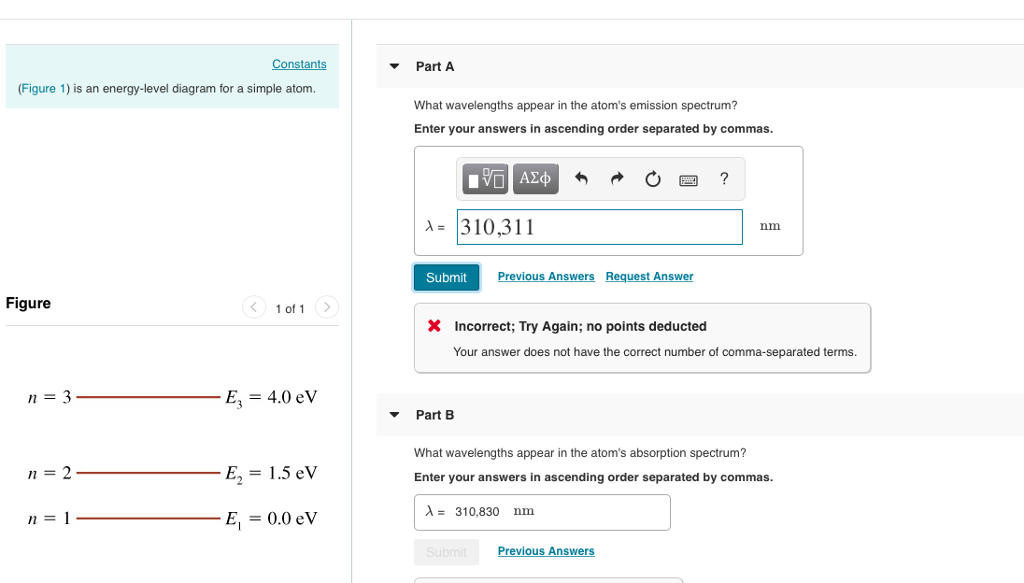
The figure is an energy-level diagram for a simple atom. (figure 1)
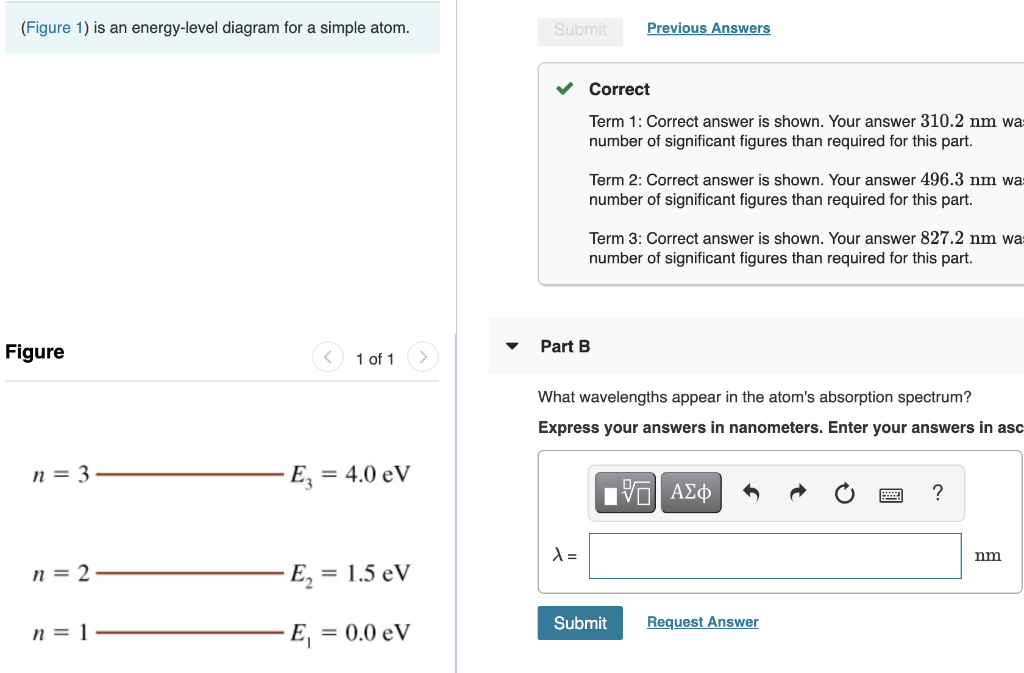
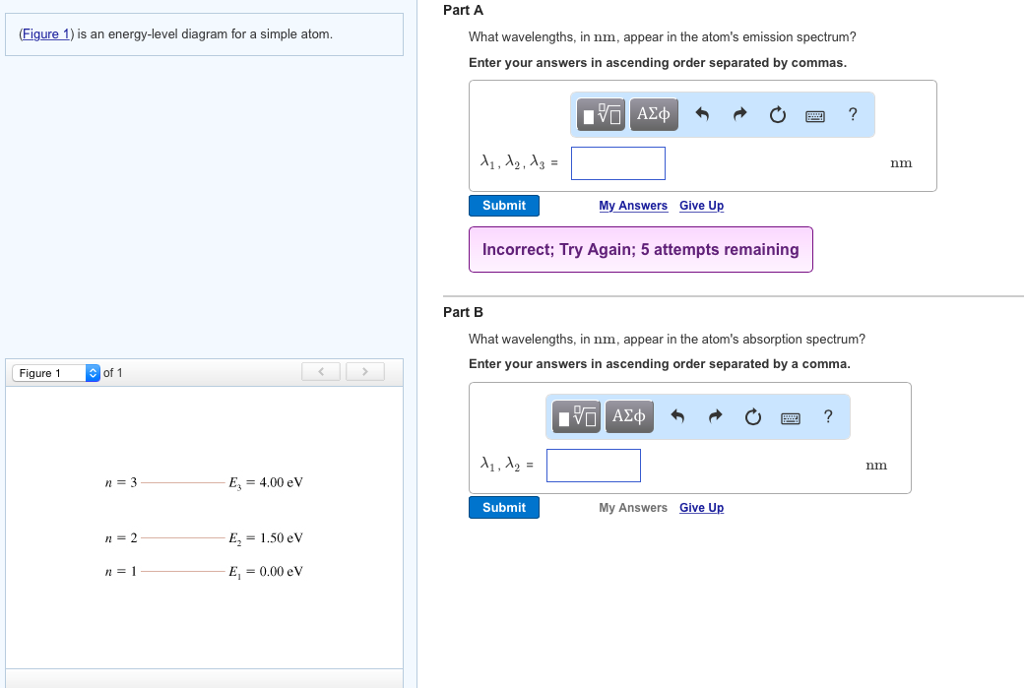
The figure (Figure 1) is an energy-level diagram for a simple atom. What wavelengths, in nm, appear in the atom's emission spectrum? Enter your answers in ascending order separated by commas. What wavelengths, in nm, appear in the atom's absorption spectrum? Enter your answers in ascending order separated by commas. The angular momentum of an electron in a Bohr's orbit of H-atom is 3.1652×10−34 kg.m2/sec. The wavenumber in terms of Rydberg constant (R) of the spectral line ...1 answer · Top answer: The spectral lines 4, 5 and 6 will not occur in the absorption spectrum. They are for the transitions from the higher excited state to lower excited state ... We like representing these energy levels with an energy level diagram. The energy level diagram gives us a way to show what energy the electron has without having to draw an atom with a bunch of circles all the time. Let's say our pretend atom has electron energy levels of zero eV, four eV, six eV, and seven eV.
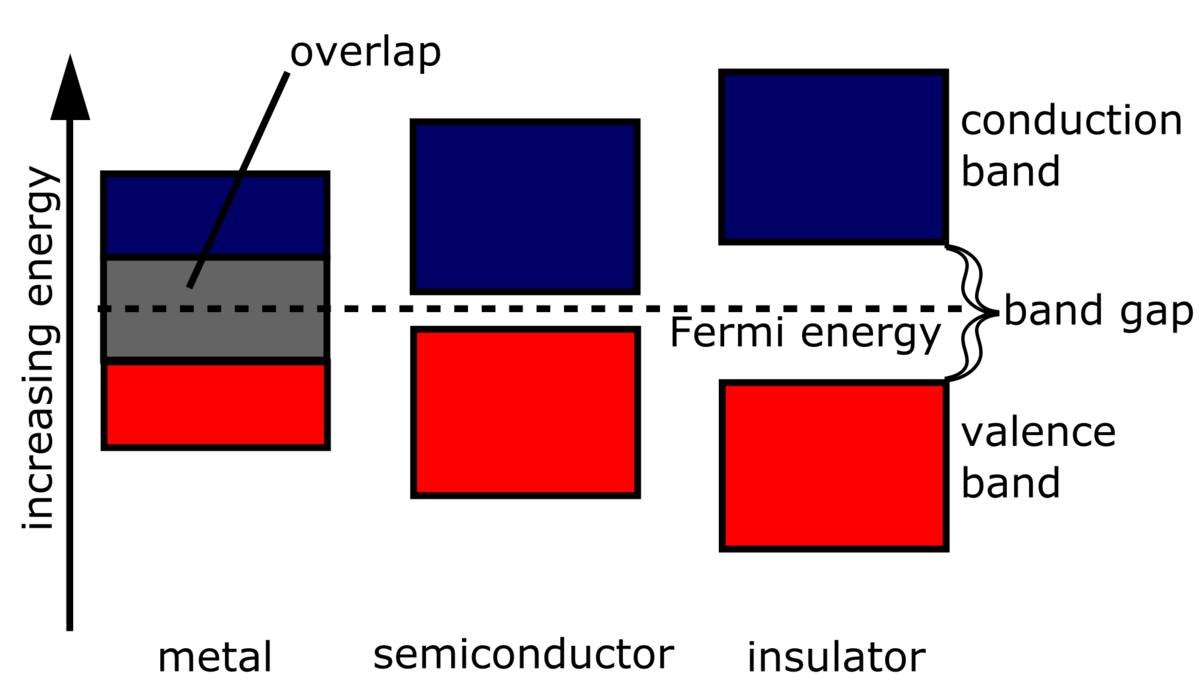
The figure is an energy-level diagram for a simple atom. (figure 1). An energy-level diagram for a hypothetical atom is shown above. a. Determine the frequency of the lowest energy photon that could ionize the atom, initially in its ground state. b. Assume the atom has been excited to the state at -1.0 electron volt. i. Determine the wavelength of the photon for each possible spontaneous transition. ii. 02.08.2021 · Figure 2.5.10 Orbital Energy Level Diagram for a Typical Multielectron Atom. Because of the effects of shielding and the different radial distributions of orbitals with the same value of n but different values of l, the different subshells are … 2- 1 149 (very long) 0 O2 2 120.7 2 The bond distance in N2 2- is very close to the expected bond distance for a diatomic with 12 valence electrons, as shown in Figure 5.8. 5.6 The energy level pattern would be similar to the one shown in Figure 5.5, with the interacting orbitals the 3s and 3p rather than 2s and 2p. We then present a simple diagram containing only the most important features and discuss the temperature and doping dependence of the energy bandgap. 2.3.3.1 Energy band diagrams of common semiconductors . The energy band diagrams of semiconductors are rather complex. The detailed energy band diagrams of germanium, silicon and gallium arsenide are shown in …
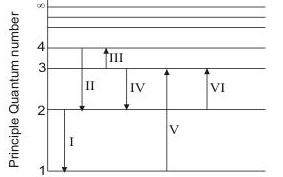
Physics questions and answers. (Figure 1) is an energy-level diagram for a quantum system. Part A What wavelengths appear in the system's emission spectrum? Express your answers in nanometers separated by commas. VO ΑΣΦ ? 828.75,310.78,407.25 nm Figure 1 of 1 Submit Previous Answers Request Answer X Incorrect; Try Again; 4 attempts remaining ... Imgur. The energy of the electron of a monoelectronic atom depends only on which shell the electron orbits in. The energy level of the electron of a hydrogen atom is given by the following formula, where. n. n n denotes the principal quantum number: E n = − 1312 n 2 kJ/mol. E_n=-\frac {1312} {n^2}\text { kJ/mol}. E n. For this question consider the figure to the right that shows an energy level diagram for a certain atom (not hydrogen). Several transitions are shown and labeled by letters. Note the diagram is not drawn to scale. ii) What wavelengths appear in the atom's absorption spectrum? (Enter your wavelengths in the order of smallest to largest.) Atomic spectrum: The atomic ...1 answer · Top answer: Given data: • The energy of the first level is E1=0.00eVE1=0.00eV • The energy of the second level is E2=1.57eVE2=1.57eV • The energy of the third ...
Equal emphasis is given to experimental results and theoretical calculations to figure out the structure-function correlation between the active-sites configuration and the intrinsic electrocatalytic performance. A research paradigm is suggested to design advanced single-metal-atom catalysts for fuel cells and metal-air batteries. Regardless of these developments, we … For this question consider the figure to the right that shows an energy level diagram for a certain atom (not hydrogen). Several transitions are shown and labeled by letters. Note the diagram is not drawn to scale. (Figure 1) is an energy-level diagram for a simple atom. 2 Revie Part A What wavelengths, in nm, appear in the atom's emission spectrum? Enter your answers in ascending order separated by commas. View Avaliable Hint(s) IVO AED ? 11. A2, A3 - 5.41 nm Submit Previous Answers Figure 1 of 1 Part B What wavelengths, in nm, appear in the atom's ... Thus the energy required to move an electron from the K shell to the L shell in an atom is KB - LB (with slight differences for different L shell substates). Binding energies and energy differences are sometimes displayed on an energy level diagram. Figure 1-2 shows such a diagram for the K and L shells of the element iodine.
Transcribed image text: (Figure 1) is an energy-level diagram for a simple atom. Part A What wavelengths appear in the atom's emission spectrum? Express your answers in nanometers. Enter your answers in ascending order separated by commas.
Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in
In the formation of a simple covalent bond, each atom supplies one electron to the bond - but that doesn't have to be the case. ... What all these have got in common is active lone pairs of electrons in the outer energy level. These are used to form co-ordinate bonds with the metal ion. All ligands are lone pair donors. In other words, all ligands function as Lewis bases. Bonding in …
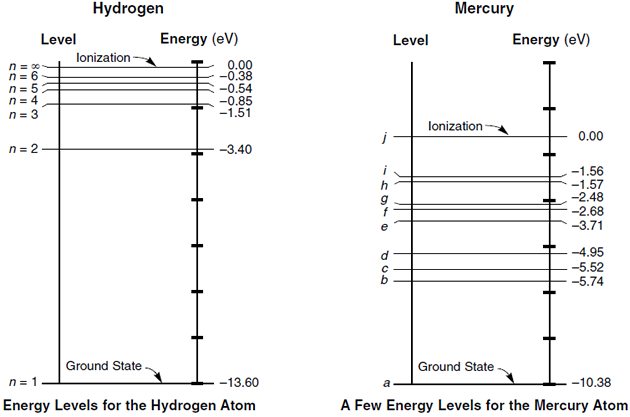
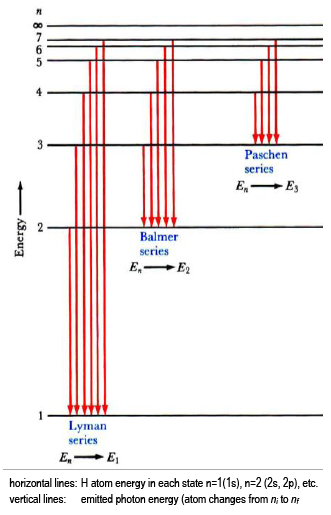
Refer to Figure 1 when answering the first 7 questions of this exam. 1. Which series of electron transitions in the energy-level diagram for Hydrogen produce the lines shown in the absorption-line spectrum of Hydrogen? ANSWER. Series #2 2. Which series of electron transitions in the energy-level diagram produce the "Balmer"
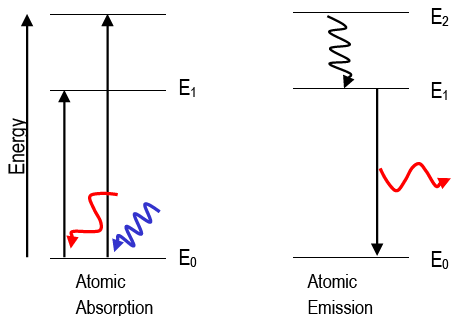
39.50. Model: Photons are emitted when an atom undergoes a quantum jump from a higher energy level to a lower energy level. On the other hand, photons are absorbed in a quantum jump from a lower energy level to a higher energy level. Because most of the atoms are in the n = 1 ground state, the only quantum jumps in the absorption spectrum start from the n = 1 state.
The energy level diagram for this reaction is shown in Figure 3 . Figure 3 ... To get full marks you should use the words atom, electron and ion in your answer. ... (1) 3 (ii)€€€€€Calculate the energy released in the reaction.
The figure is an energy-level diagram for a simple atom. What wavelengths appear in the atom's emission spectrum? #1: From wavelength 3 to 1. #2: From wavelength 3 to 2. #3: From wavelength 2 to 1. What wavelengths appear in the atom's absorption spectrum?
Powered by FlexBook® textbook Platform ® © CK-12 Foundation 2022; Please wait... Please wait...
The diagram above shows part of an energy-level diagram for a certain atom. The wavelength of the radiation associated with transition A is 600 nm (1 nm = 1 x 10 -9 m) and that associated with transition B is 300 nm. a. Determine the energy of a photon associated with transition A. b. Determine the λ of the radiation associated with transition C
Question: (Figure 1) is an energy-level diagram for a simple atom. What wavelengths appear in the atom's emission spectrum? Enter your answers in ascending order separated by commas. What wavelengths appear in the atom's absorption spectrum? Enter your answers in ascending order separated by commas.
Note: Figure not to scale For a correct energy-level diagram with horizontal lines and -6 eV at the bottom 1 point Note: The energy-level diagram does not need to be drawn to scale in order to earn this point. For correct labeling of energy levels and quantum numbers (e.g., n = 1 correlated with
• Energy-level diagram; A pictorial level diagram designation of where electrons are placed in an electron configuration. For example, the energy-level diagram for the ground-state electron configuration of carbon is 1s2 2s2 2p2. For chlorine: 1s2 2s2 2p6 3s2 3p5. Electron Configuration of Atoms 4 1s 2s 2p Energy-level diagram for chlorine ...
A part of the energy-level diagram for carbon monoxide is shown in Figure VII-4. The energy levels have been obtained by analysis of the observed frequencies of the lines in the emission and absorption spectra of the molecule. It has a node between each bonded pair of carbons and is antibonding. Figure 1.18 shows these tt MO s in an energy-level diagram. .
PhysicsLAB: Energy-Level Diagrams. Energy level diagrams are a means of analyzing the energies electrons can accept and release as they transition from one accepted orbital to another. These energies differences correspond to the wavelengths of light in the discreet spectral lines emitted by an atom as it goes through de-excitation or by the ...
Figure Ex38.22 is an energy-level diagram for a simple atom, inwhich E1 = 0.0 eV, E2 =1.2 eV, and E3 =3.3eV. Calculate the wavelength(s)which appear(s) in the following two cases, in each of which youshould submit all of the wavelength(s) in the singleanswer box,separated by spaces.Figure Ex38.22(a) the atom's emission spectrum nm(b) the atom's ...
The index n is called the energy quantum number or principal quantum number.The state for is the first excited state, the state for is the second excited state, and so on. The first three quantum states (for of a particle in a box are shown in .. The wave functions in are sometimes referred to as the “states of definite energy.” Particles in these states are said to occupy energy levels ...
The Gibbs free energy diagram is illustrated in Figure S4. ... N 1 atom refers to the N atom bonded to TM atom, and the N 2 atom is the end N atom. The U L of NRR on W- and Mn-VSe 2 are quite different though both their PLSs are the first hydrogenation step. Thus, the activation of N 2 molecule was further investigated in terms of electronic structure. As shown in Fig. 6, the …
Each atom of the impurity fits in four germanium atoms as shown in the figure above. Hence, each Arsenic atom provides one free electron in the Germanium crystal. Since an extremely small amount of arsenic, impurity has a large number of atoms; it provides millions of free electrons for conduction. Energy Diagram of n-Type Semiconductor. The Energy level diagram of the n …
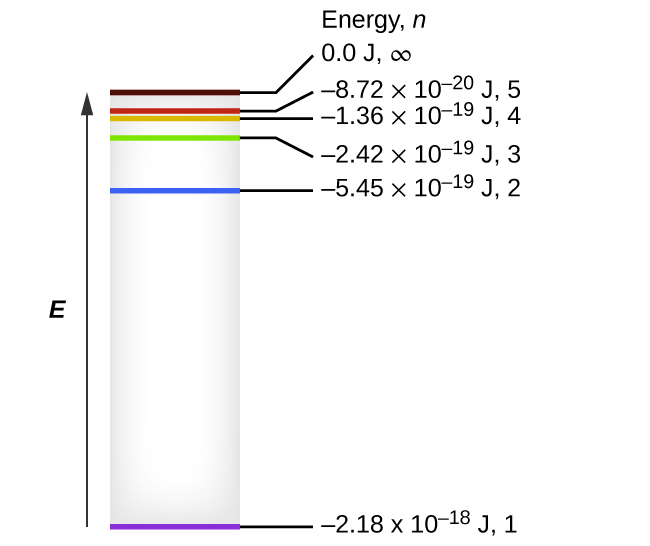
30.09.2021 · Figure \(\PageIndex{6}\) shows that the energy levels become close\(r\) and close\(r\) togethe\(r\) as the value of n increases, as expected because of the 1/n 2 dependence of orbital energies. Figure \(\PageIndex{6}\): Orbital Energy Level Diagram fo\(r\) the Hydrogen Atom with a single electron. Each box corresponds to one orbital. Note that ...
We like representing these energy levels with an energy level diagram. The energy level diagram gives us a way to show what energy the electron has without having to draw an atom with a bunch of circles all the time. Let's say our pretend atom has electron energy levels of zero eV, four eV, six eV, and seven eV.
The angular momentum of an electron in a Bohr's orbit of H-atom is 3.1652×10−34 kg.m2/sec. The wavenumber in terms of Rydberg constant (R) of the spectral line ...1 answer · Top answer: The spectral lines 4, 5 and 6 will not occur in the absorption spectrum. They are for the transitions from the higher excited state to lower excited state ...
The figure (Figure 1) is an energy-level diagram for a simple atom. What wavelengths, in nm, appear in the atom's emission spectrum? Enter your answers in ascending order separated by commas. What wavelengths, in nm, appear in the atom's absorption spectrum? Enter your answers in ascending order separated by commas.














0 Response to "37 the figure is an energy-level diagram for a simple atom. (figure 1)"
Post a Comment