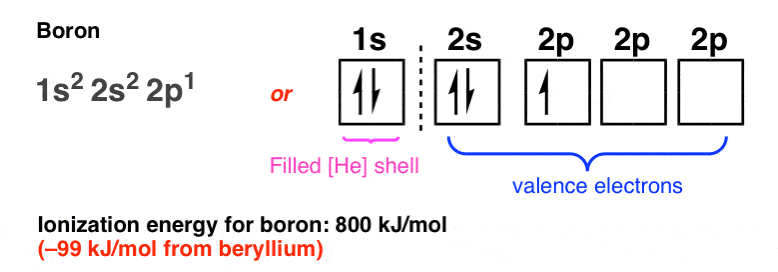
37 orbital filling diagram for boron
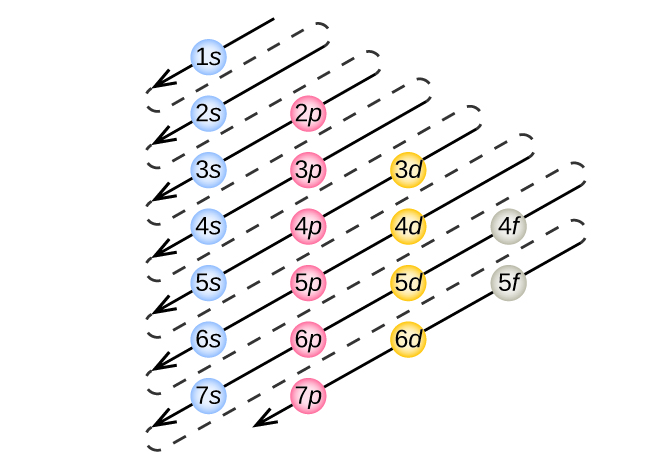
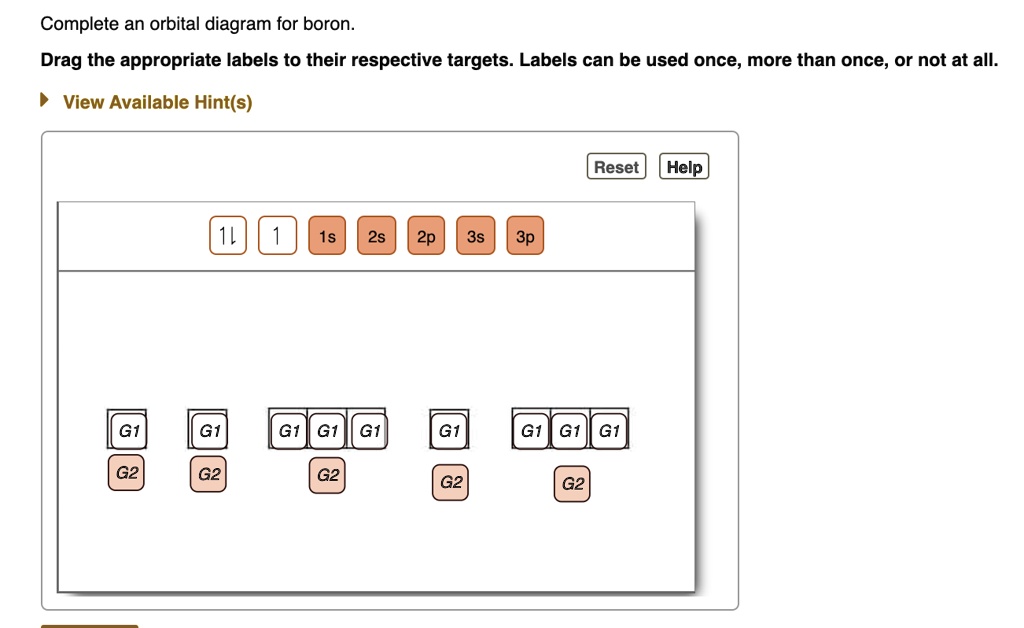
Hexagonal boron nitride (h-BN), a layered material isostructural to graphite, has similar exotic properties like graphite. With single atom thick and alternating boron and nitrogen atoms in its atomic structure, h-BN is an insulator with band gap ~ 5.9 eV. An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom. In an orbital filling diagram, the individual orbitals are shown as circles (or squares) and orbitals within a sublevel are drawn next to each other horizontally. Show transcribed image text Draw an orbital diagram for boron.
(quicker to draw than orbital filling diagrams) 1 s2 2s2 2p4 3. Electron Dot shows only the valence (outer energy level) electrons EX. Oxygen atom 1 . Write orbital filling diagrams, electron configurations, and electron dot diagrams for the following elements. LKrl 10 Table. a. Boron b. Silicon c. Sulfur d. Calcium e. Iodine f. Rubidium g ...

Orbital filling diagram for boron
Orbital Filling Diagrams. An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom. In an orbital filling diagram, the individual orbitals are shown as circles (or squares) and orbitals within a sublevel are drawn next to each other horizontally. Boron (B) electron configuration with full orbital diagram. Boron is the 5th element in the periodic table and the first element in group-13. The atomic number of boron is 5 and its symbol is 'B'. The standard atomic mass of boron is 10.806. The period of boron is 2 and it is a p-block element. This article gives an idea about the electron ... Orbital filling diagrams essentially just turn this big list of electron locations . In the same way, the orbital filling diagram for nitrogen will be. Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s.
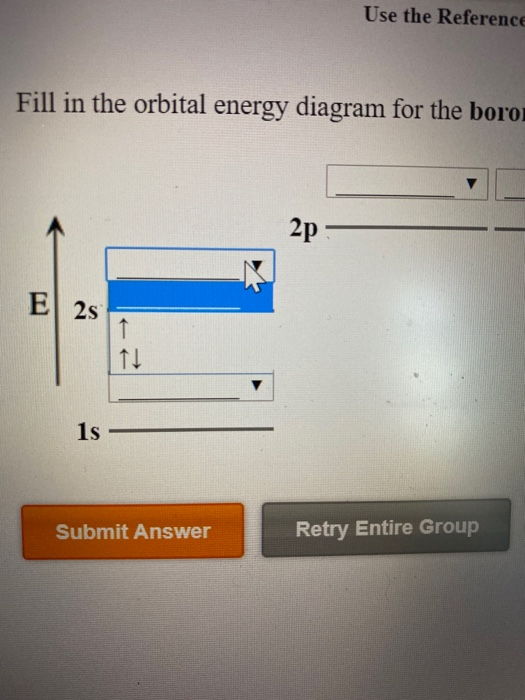
Orbital filling diagram for boron. You may need to draw the orbital filling diagram for the last sublevel that contains electrons. 0 The number of unpaired electrons in an atom can be determined from the orbital filling diagrams and correctly applying Hund's rule. Boron has one unpaired electron, while carbon has two, and nitrogen has three. The first transition series represents the filling of what orbital? the 3d orbital How many dots would a Lewis dot diagram for Boron have? The Lewis dot diagram for Boron has three dots. What is... Relating orbital filling to the Periodic Table Note: On some screens the V for vanadium (element 23) may look a bit like a Y. This isn't a mistake, but an effect of converting my original diagram into a lower quality gif image for efficient web use. Mar 18, 2019 · Show the orbital-filling diagram for N (nitrogen). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Use the buttons at the top of the tool to add %(8). Question: Orbital Diagrams Draw an orbital diagram for boron. Use this tool to draw the orbital diagram.
1.1.2022 · Hexagonal boron nitride (h-BN) as a type of two-dimensional (2D) materials has gained significant attention in green energy applications recently. In the past, h-BN has mainly been regarded as inert materials because of the poor conductivity and is … Hey there! We have to draw an orbital filling diagram for boron. We can determine the ground-state electron configuration of boron (B) by locating its position in the periodic table. Ground-state means that the element is in its lowest energy form (not in excited state). Orbital filling diagrams | The Cavalcade o' Chemistry Feb 23, 2016 · The orbital filling diagram of boron. I skipped past beryllium because I was getting bored. The electron configuration of boron is 1s²2s²2p¹, which means that there are two electrons in the 1s orbital, two electrons in the 2s orbital, and one electron in the 2p orbitals. 1. Orbital Filling Diagram 02 Ex. 2, Electron Configuration 02 Ex. (gives the most information) Is (quicker to draw than orbital filling diagrams) Dot Pb 3. Electron Dot shows only the valence (outer energy level) electrons Oxygen atom Ex. 1. Write orbital filling diagrams, electron configurations, and electron dot diagrams for the following ...
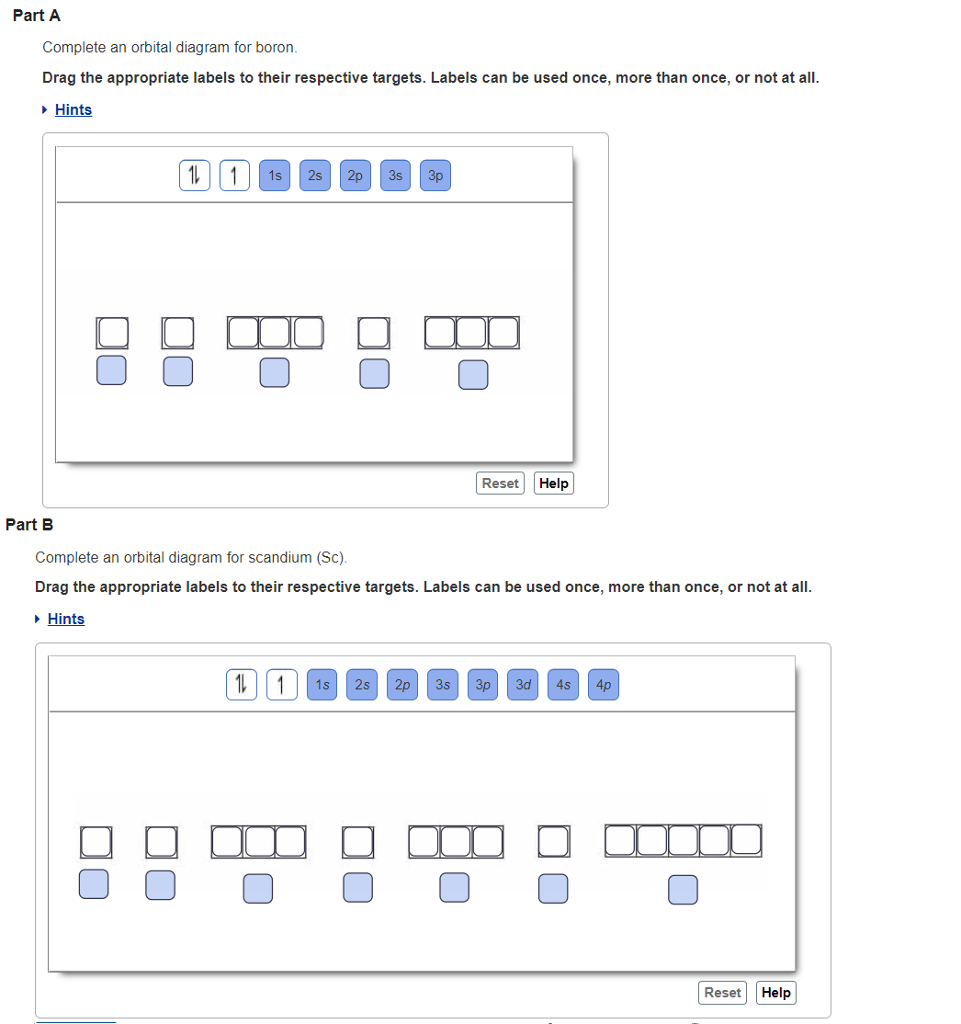
orbital diagram (orbital box diagram) : 1s box has 2 arrows (as per helium above), 2s box has 2 arrows as per boron above, but now we see that there are 3 orbitals that make up the p-subshell (p x, p y, p z), into which we need to place 4 arrows. The molecular orbital diagram representing this order of energy levels is shown in fig. Fig. No. 5 Order of Energy Levels for Boron, Carbon, Nitrogen etc. This kind of energy reversal is due to mixing of 2s and 2p orbitals where the energy difference is very close, that is, for B, C, and N … Problem: Part A. Complete an orbital diagram for boron.Draw orbital diagrams, and use them to derive electron configurationsTo understand how to draw orbital diagrams, and how they are used to write electron configurations.The electron configuration of an element is the arrangment of its electrons in their atomic orbitals. Electron configurations can be used to predict most of the chemical ... 1. Write orbital filling diagrams, electron configurations, and electron dot diagrams for the following elements. Table: Element Orbital Filling Diagram Electron Configuration Electron Dot Diagram a. Boron b. Silicon c. Sulfur d. Calcium e. Iodine f. Rubidium g. Chromium h. Gallium
Orbital Filling Diagram Electron Configuration Electron Dot Diagram a. Boron b. Silicon c. Sulfur d. Calcium e. Iodine f. Rubidium g. Chromium h. Gallium Where are the Electrons? Write the full electron configuration, short-hand electron configuration, and fill in the orbital . Place the ending configuration and the dot diagram for the above ...
Here is an example of orbital configuration for Hydrogen, Helium and Carbon. The simplest atom hydrogen has 1 electron. It will go into the 1s orbital with a spin in either direction. We can represent this with either an orbital filling diagram or an electron configuration. The next atom is helium. It has 2 electrons.
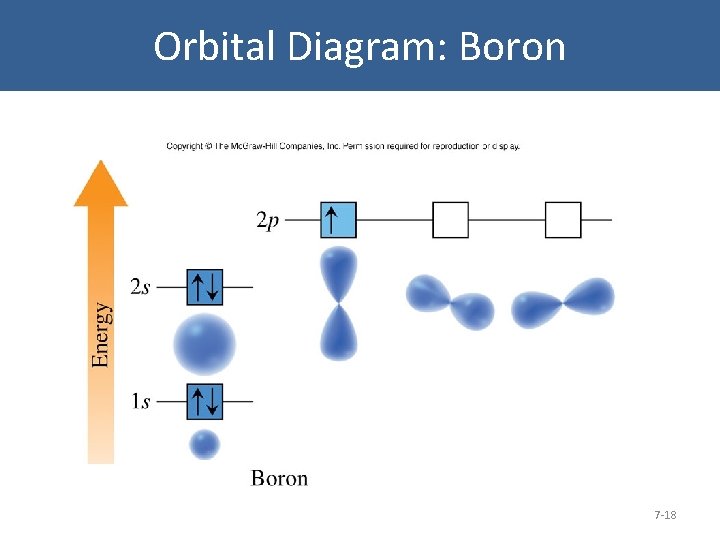
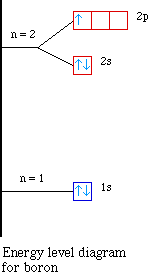
The orbital diagram for boron as shown has the one electron in the 2p orbital. The electron can be placed in any of the three 2p orbitals. The electron configuration for boron is 1s 2 2s 2 2p 1. The energy level diagram for boron is show below. For the next element, carbon, the sixth electron must be placed in the correct orbital.
1. Write orbital filling diagrams, electron configurations, and electron dot diagrams for the following elements. Table: Element Orbital Filling Diagram Electron Configuration Electron Dot Diagram a. Boron b. Silicon c. Sulfur d. Calcium e. Iodine f. Rubidium g. Chromium h. Gallium
#1. Draw the MO diagram for `B_2`. First step is to determine which MO diagram we're using. In this case, we're using the standard one. Draw out the MO diagram and label in the valence electrons. Boron has 2 electrons in the `2s` orbitals and 1 electron in the `2p` orbital. That's it for the MO diagram of `B_2`!
The electron configuration of boron is 1s²2s²2p¹, which means that there are two electrons in the 1s orbital, two electrons in the 2s orbital, and one electron in the 2p orbitals. This gives us an orbital filling diagram of. The next element is beryllium which has four electrons. The orbital diagram for beryllium is shown here.
Feb 23, 2016 · The orbital filling diagram of boron. I skipped past beryllium because I was getting bored. The electron configuration of boron is 1s²2s²2p¹, which means that there are two electrons in the 1s orbital, two electrons in the 2s orbital, and one electron in the 2p orbitals. This gives us an orbital filling diagram of:
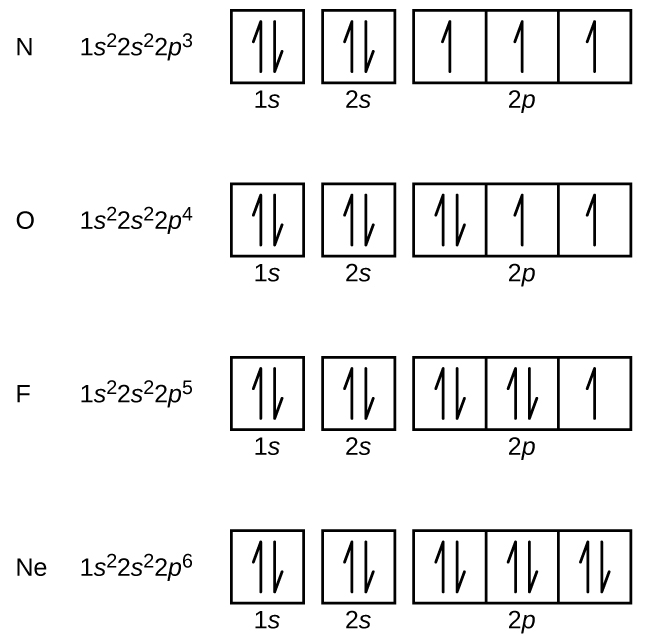
The number of unpaired electrons in an atom can be determined from the orbital filling diagrams and correctly applying Hund's rule. Boron has one unpaired electron, while carbon has two, and nitrogen has three. How many unpaired electrons does fluorine have? 1.
Show the orbital-filling diagram for N (nitrogen). Stack the subshells in order of; Question: Draw an orbital diagram for boron. Use this tool to draw the orbital diagram. Draw an orbital diagram for scandium (Sc). Use this tool to draw the orbital diagram. How many orbitals are there in the third shell (n = 3)? Express your answer numerically ...
The orbital filling diagram of boron. I skipped past beryllium because I was getting bored. The electron configuration of boron is 1s²2s²2p¹, which means that there are two electrons in the 1s orbital, two electrons in the 2s orbital, and one electron in the 2p orbitals. This gives us an orbital filling diagram of: This problem has been solved!
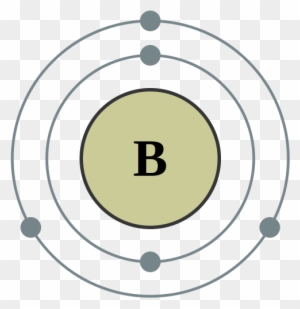
Thus, the electron configuration and orbital diagram of lithium are: An atom of the alkaline earth metal beryllium, with an atomic number of 4, contains four protons in the nucleus and four electrons surrounding the nucleus. The fourth electron fills the remaining space in the 2s orbital. An atom of boron (atomic number 5) contains five electrons.
An orbital diagram is used to determine an atom’s electron configuration. There are guidelines for determining the electron configuration of an ... Filling in an Aufbau Diagram. ... the filling order is less clear for degenerate sublevels. For example, for boron through neon, the electron filling order of the 2p orbitals follows Hund’s Rule.
Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13 ...
Boron is the fifth element with a total of 5 electrons. In writing the electron configuration for Boron the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for B goes in the 2s orbital. The remaining electron will go in the 2p orbital.
Orbital filling diagrams essentially just turn this big list of electron locations . In the same way, the orbital filling diagram for nitrogen will be. Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s.
Boron (B) electron configuration with full orbital diagram. Boron is the 5th element in the periodic table and the first element in group-13. The atomic number of boron is 5 and its symbol is 'B'. The standard atomic mass of boron is 10.806. The period of boron is 2 and it is a p-block element. This article gives an idea about the electron ...
Orbital Filling Diagrams. An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom. In an orbital filling diagram, the individual orbitals are shown as circles (or squares) and orbitals within a sublevel are drawn next to each other horizontally.



















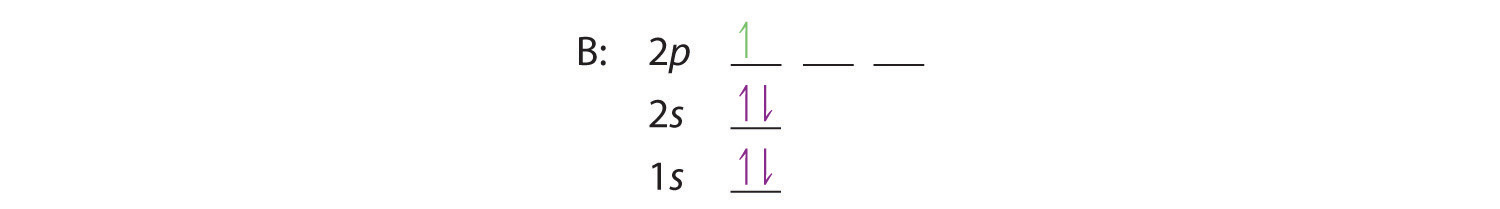


0 Response to "37 orbital filling diagram for boron"
Post a Comment