39 copper gold phase diagram
This article is a compilation of ternary alloy phase diagrams for which copper (Cu) is the first-named element in the ternary system. The other elements are Fe, Mn, Ni, Pb, S, Sb, Si, Sn, Ti and Zn. The diagrams are presented with element compositions in weight percent. The article includes 42 phase diagrams (liquidus projection, solidus ... Solution The copper-gold phase diagram is constructed below. 9 Cite the phases that are present and the phase compositions for the following alloys: (a) 15 wt% Sn–85 wt% Pb at 100°C (212°F) (b) 25 wt% Pb–75 wt% Mg at 425°C (800°F) (c) 85 wt% Ag–15 wt% Cu at 800°C (1470°F)
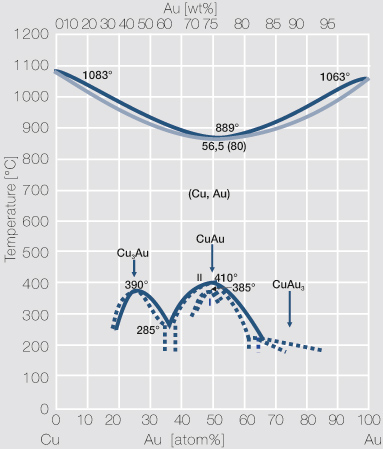
by PP Fedorov · 2016 · Cited by 35 — Kurnakov discovered two ordered phases in the gold–copper system, one based on CuAu and the other on Cu3Au. Later, one more phase having ideal.
Copper gold phase diagram
This zone is represented at the left of the diagram where the alloy is in solid solution (phase ), a face centred cubic structure like copper. For the typical alloy CuBe1.9 (1.8 to 2% of beryllium) the alloy is in the phase between 720°C and 860°C. For industrial purposes the solution heat treatment is done between 750° and 800°C. phase diagrams. Copper-gold, which forms a continuous range of fcc solid solutions at elevated temperatures, has been exploited since ancient times [6] due to its natural occurrence and attractive reddish colour. Its binary phase diagram has been intensively investigated, primarily because its high temperature α-(Au,Cu) solid solution undergoes Eutectic phase diagram for a silver-copper system. 2800 2600 2400 2200 2000 1800 1600 MgO CaO 20 40 60 80 100 0 C) L MgO ss + L MgO ss CaO ss + L CaO ss MgO ss + CaO ss Wt % Eutetic phase diagram for MgO-CaO system. Temperature (Lecture 19 – Binary phase diagrams 4 of 16 11/23/05
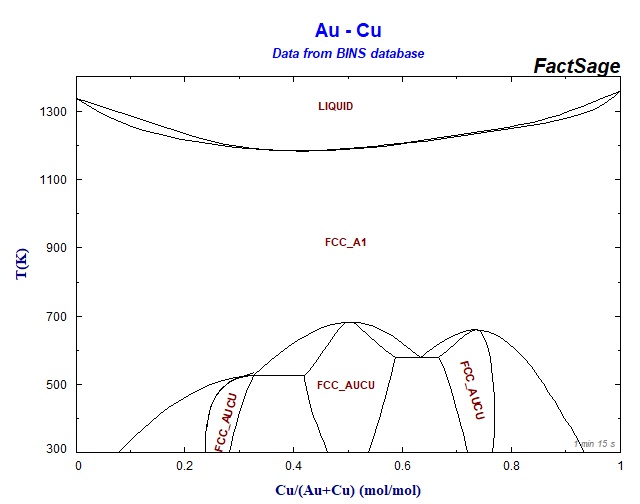
Copper gold phase diagram. Copper in Powder Metallurgy. A. Phase Diagrams 1. Iron-Copper System The iron-copper phase diagram, taken from Hansen,3 is presented in Figure 1. Hansen3 also gives a thorough review of the work done on the system up to 1957. A review of more recent work, up to 1963 was done by . Elliott~ The most recent version of the phase diagram An intriguing feature of our phase diagram version is the absence of intermetallics that are analogues of the ordered phase existing in the copper-gold system, namely, a CuPd tetragonal phase and a CuPd 3 cubic phase. In the copper-palladium system these phases are dismissed by a CuPd phase, which has another structure-building principle. Homework Solutions November 17, 2005 10.7 The copper-gold phase diagram is constructed below. 10.8 This problem asks that we cite the phase or phases present for several alloys at specified temperatures. (a) For an alloy composed of 15 wt% Sn-85 wt% Pb and at 100 ... Metals like silver and gold have a difference of 0.2%; nickel and copper of 2.7%, and show complete solid solubility. But zinc and copper have 4.2% difference with maximum solubility of 38.4 wt.% Zn. (other factors are less favourable); Cadmium in copper with 16.5% size difference shows a solid solubility of 1.7 wt.%.
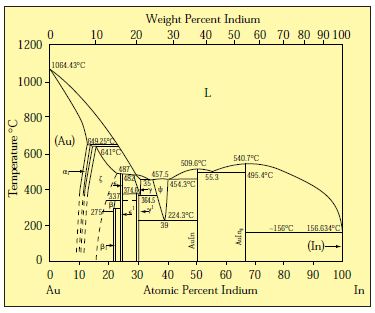
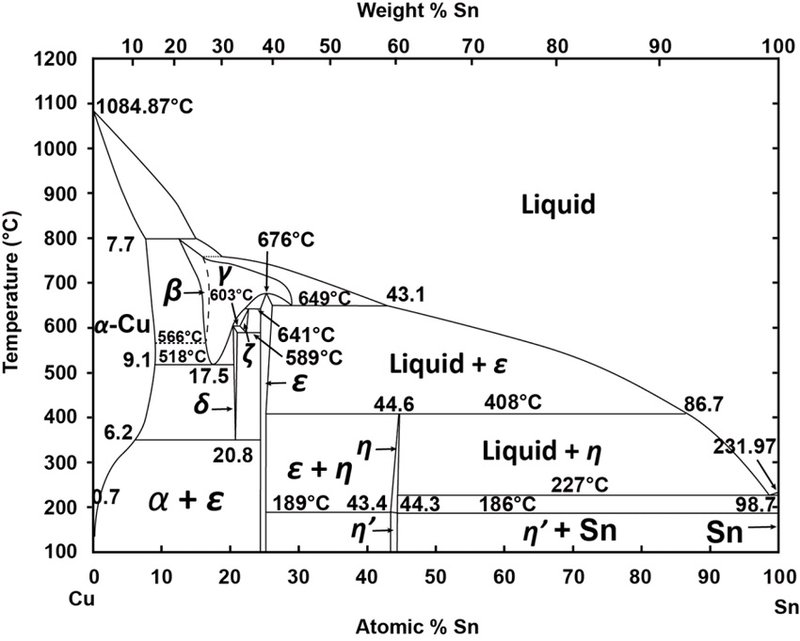
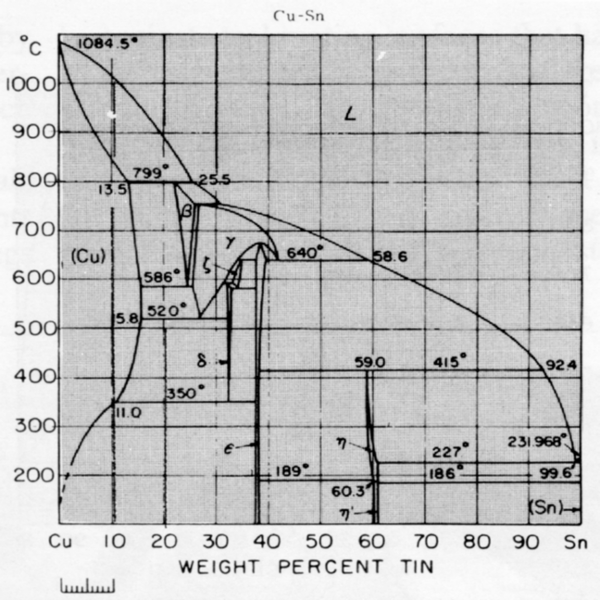
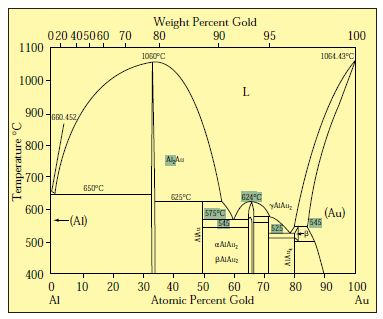
Gold-Copper Binary Alloy Phase Diagram (based on 1963 Marchinkowski M.J.). Author: Marchinkowski M.J.; Zwell L. | Document Download | Product code: ... Given here are the solidus and liquidus temperatures for the Copper-Gold system. Construct the phase diagram for this system and label each region. %Gold 0 20 40 60 80 90 95 100 Liquidus Temperatures (Cº) 1085 1042 996 946 911 942 984 1064 Solidus Temperatures (CC) 1085 1019 972 934 911 928 974 1064 B. Below is the Tin-Gold phase Gold-copper nano-alloy, "Tumbaga", in the era of nano: phase diagram and segregation Nano Lett. , 14 ( 11 ) ( 2014 ) , pp. 6718 - 6726 , 10.1021/nl503584q CrossRef View Record in Scopus Google Scholar This representation is called a phase diagram. The phase diagrams of some binary systems relevant to bronze show the behavior of alloying elements that typically results in one of the three cases mentioned previously. The copper-tin equilibrium phase diagram (Figure 3) illustrates Cases (1) and (2).
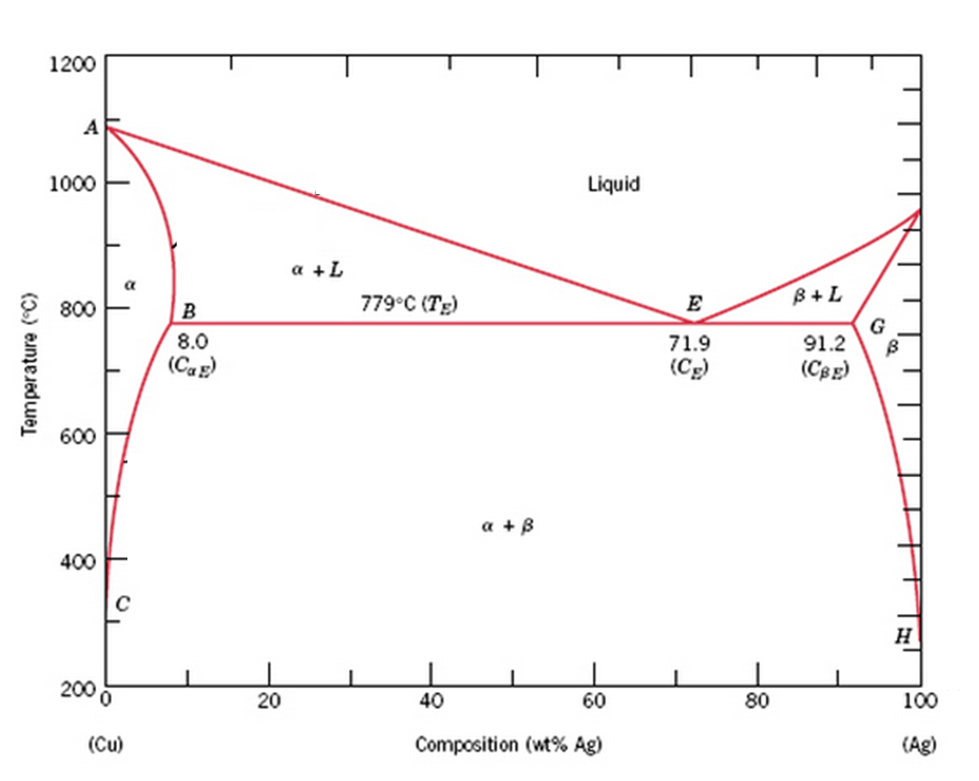
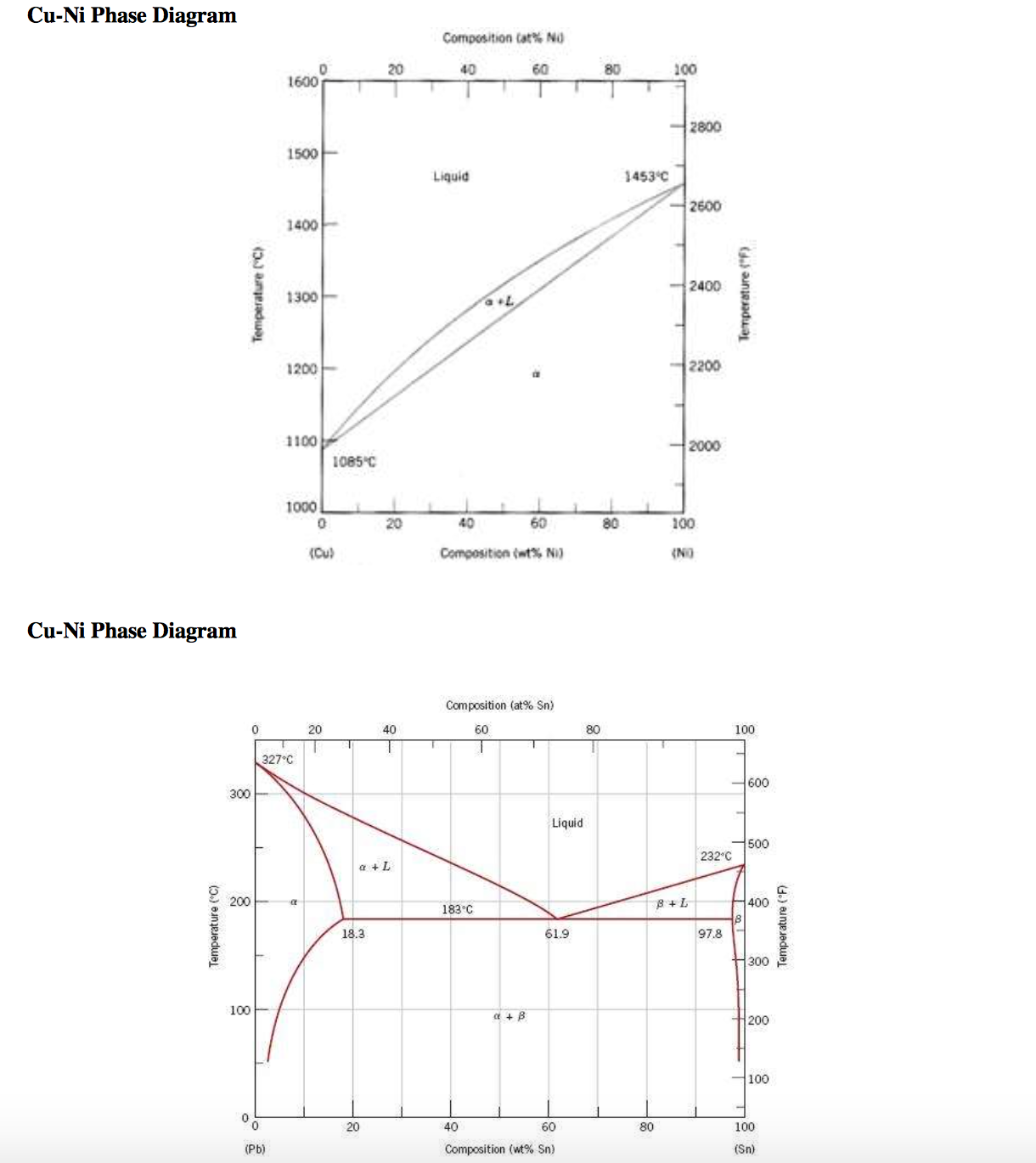
the thermal equilibrium diagram for the alloy of Copper and Nickel. In order to find what temperature 60% copper solidifies at we simply draw a vertical line from 60% copper until it hits the solidus line and at this is the point where 60% Copper has fully solidified. 0 100 10 90 20 80 30 70 40 60 50 50 60 40 70 30 80 20 90 10 100 0 900 1000 ... Gold–aluminium phase diagram. A gold–aluminium intermetallic is an intermetallic compound of gold and aluminium that occurs at contacts between the two metals. These intermetallics have different properties than the individual metals, which can cause problems in wire bonding in microelectronics. The main compounds formed are Au 5 Al 2 ... The phase diagrams of Au-Cu nanoalloys were thermodynamically investigated. ... higher copper concentration and the gold tends to enrichment on the surface. Download scientific diagram | Cu-Ag Phase Diagram. The eutectic composition is 28.1 wt% Cu-71.9 wt% Ag and the solid solubility limit of silver in copper is 8 wt% Ag (after Hansen and Anderko 1958 ...
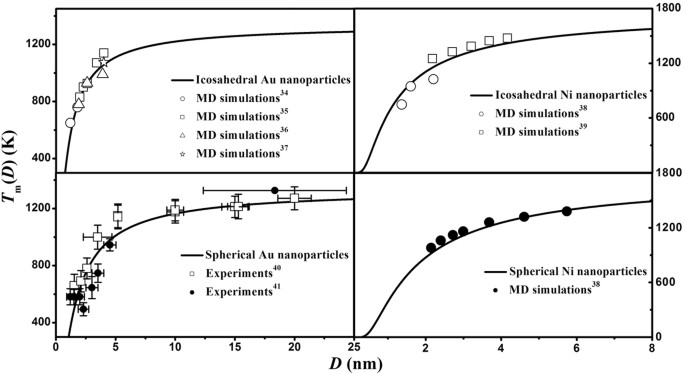
by G Guisbiers · 2014 · Cited by 125 — All phase diagrams, presented in Figure 2 at two distinct sizes (10 and 4 nm), show that the liquid region is enlarged and the solid solution ...
Mechanical Engineering questions and answers. Given here are the solidus and liquidus temperatures for the copper gold system. Construct the phase diagram for this system and label each region. (You can use excel or matlab to plot your phase diagram) Question: Given here are the solidus and liquidus temperatures for the copper gold system.
Au-Cu Phase Diagram. a Prokhorov General Physics Institute, Russian Academy of Sciences, ul. Vavilova 38, Moscow, 119991 Russia. nab. Makarov a 2, St. Petersburg, 199034 Russia. been plotted for ...
Abstract. Phase equilibria have been extrapolated to low temperatures, and a condensed phase diagram has been plotted for the Au-Cu system to be consistent with the third law of thermodynamics. Download to read the full article text.
Phase Diagrams • Indicate phases as function of T, Co, and P. • For this course:-binary systems: just 2 components.-independent variables: T and Co (P = 1 atm is almost always used). • Phase Diagram for Cu-Ni system Adapted from Fig. 9.3(a), Callister 7e. (Fig. 9.3(a) is adapted from Phase Diagrams of Binary Nickel Alloys , P. Nash
Phase diagram of gold-copper: You cannot overwrite this file. File usage. The following 2 pages link to this file: Gold Based Materials; Werkstoffe auf Gold-Basis; Metadata. This file contains additional information, probably added from the digital camera or scanner used to create or digitize it. If the file has been modified from its original ...
10.4 Given here are the solidus and liquidus temperatures for the copper–gold system. Construct the phase diagram for this system and label each region. Composition Solidus Liquidus (wt% Au) Temperature (°C) Temperature (°C) 0 1085 1085. 20 1019 1042. 40 972 996. 60 934 946. 80 911 911. 90 928 942. 95 974 984. 100 1064 1064. Solution The ...
The system copper (Cu) - zinc (Zn). Let's start with copper (Cu) - tin (Sn). This system contains what we call "bronze". Here is the phase diagram: ...
Copper Gold Phase Diagram. Al Mg Phase Diagram. Copper Oxide Phase Diagram. Gallery of Aluminium Copper Phase Diagram. How To Stop Cats From Scratching Flea And Tick Collars Apartments Naples Fl Farmhouse Table Lamps Animal Friends Humane Society Bathroom Sink Parts Is Popcorn Good For Dogs Marriott Pet Policy Ymca Athens Ga Cat Has Dandruff ...
as axes are called phase diagrams. DEF. A phase diagram (or equilibrium diagram) is a diagram with T and composition as axes, showing the equilibrium constitution. The phase diagram of an alloy made of components A and B, for all combinations of T and X B, defines the A-B system. Binary systems have two components, ternary systems three, and so on.
Generate Phase Diagram Compositional Phase Diagram; Aqueous Stability (Pourbaix) Tags: Gold cupride (1/1) Copper gold (1/1) - L1o type Tetraauricupride Gold copper (1/1) Material Details; Final Magnetic Moment 0.001 μ B. Calculated total magnetic moment for the unit cell within the magnetic ordering provided (see below). ...
All data taken from: "Binary Alloy Phase Diagrams", 2nd Edn., eds. T. B. Massalski, H. Okamoto, P. R. Subramanian and L. Kacprzak, in 3 volumes, ASM International, Ohio, USA, 1990 Temperature, ºC 2600 2200 1800 1400 1000 600 2447ºC 1769ºC 0 10 20 30 40 50 60 70 80 90 100 Ir Platinum, wt% Pt Platinum, at% 0 10 20 30 40 50 60 70 80 90 100 ...
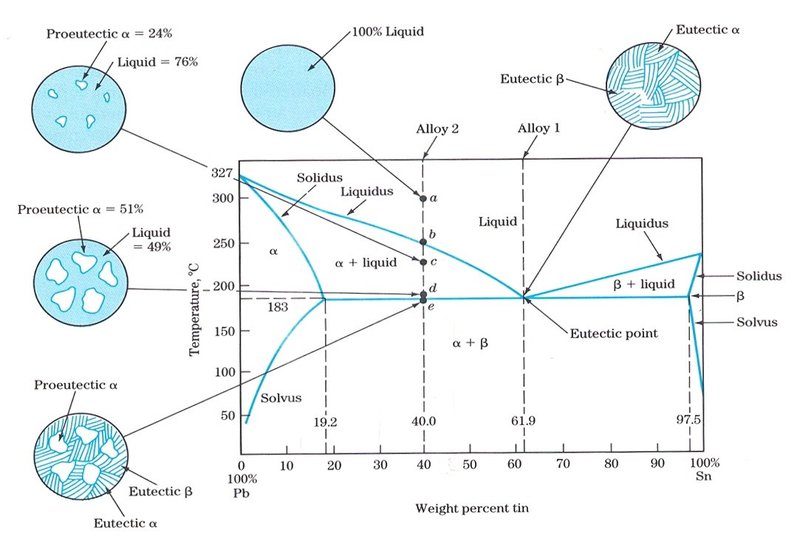
It is 19 wt% Mg-81 wt% Pb. Melting temperature of Mg2Pb 550 oC) Extensive solubility. of the phase diagrams. Eutectic transformation also called eutectic reaction. A eutectic reaction is an invariant reaction, and a eutectic point is also called an invariant point. the horizontal tie line at the eutectoid temperature is termed a eutectoid isotherm.
Copper gold imc (au3cu) Formula: Au 3 Cu; Molecular weight: 654.446; Information on this page: Notes; Data at other public NIST sites: X-ray Photoelectron Spectroscopy Database, version 4.1; Options: Switch to calorie-based units
Aluminum-Copper Phase Diagram Another commonly used phase diagram is the aluminum-copper phase diagram, which is useful for understanding precipitation strengthening in Al-Cu alloys. The amount of copper present in an alloy is plotted on the x-axis. The phase fields of interest are the Al, θ, and Al+θ phase fields on the left hand side.
Eutectic phase diagram for a silver-copper system. 2800 2600 2400 2200 2000 1800 1600 MgO CaO 20 40 60 80 100 0 C) L MgO ss + L MgO ss CaO ss + L CaO ss MgO ss + CaO ss Wt % Eutetic phase diagram for MgO-CaO system. Temperature (Lecture 19 – Binary phase diagrams 4 of 16 11/23/05
phase diagrams. Copper-gold, which forms a continuous range of fcc solid solutions at elevated temperatures, has been exploited since ancient times [6] due to its natural occurrence and attractive reddish colour. Its binary phase diagram has been intensively investigated, primarily because its high temperature α-(Au,Cu) solid solution undergoes
This zone is represented at the left of the diagram where the alloy is in solid solution (phase ), a face centred cubic structure like copper. For the typical alloy CuBe1.9 (1.8 to 2% of beryllium) the alloy is in the phase between 720°C and 860°C. For industrial purposes the solution heat treatment is done between 750° and 800°C.


![Top: part of the phase diagram of Au-Cu [1]. Bottom ...](https://www.researchgate.net/profile/John-Banhart/publication/216046001/figure/fig1/AS:670013379858440@1536755046309/Top-part-of-the-phase-diagram-of-Au-Cu-1-Bottom-electrical-resistivity-for.png)
![Quasi-binary phase diagram for 18 ct gold alloys [3]. In this ...](https://www.researchgate.net/profile/Bernd-Kieback/publication/325342903/figure/fig1/AS:629792688857089@1527165686463/Quasi-binary-phase-diagram-for-18-ct-gold-alloys-3-In-this-diagram-the-gold-content.png)



![PDF] The aluminium-copper-gold ternary system | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/49d24ee9c62e3b27012ceb94405de8d147107693/2-Figure2-1.png)


0 Response to "39 copper gold phase diagram"
Post a Comment