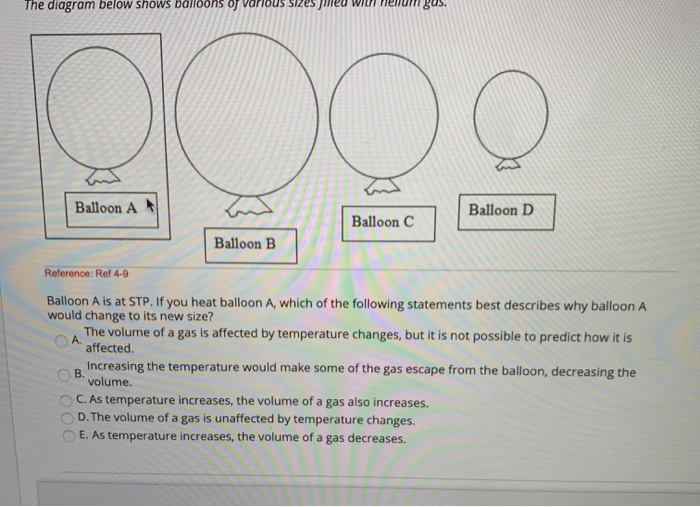
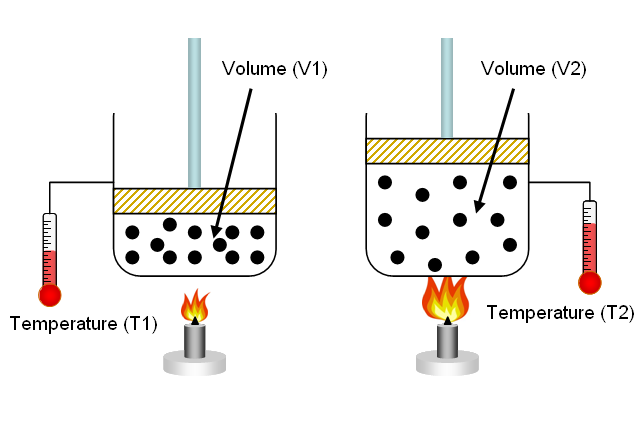
39 one way to increase the volume of the gas in the balloon in the diagram above is to -
Answers: 2 on a question: Use the following diagram for the next four questions. The particle models shown above represent a proposed two-step mechanism for the destruction of ozone (O3) in the upper atmosphere. Based on the proposed mechanism, which of the following best describes the concentration of the species represented above as the reaction occurs? A. The concentration is larger than ... The figure above makes an important point: the air molecules in a balloon ... Part C: Increasing the temperature of the gas in a balloon will cause the gas ...
25.05.2015 ... If the balloon is closed, then yes, both volume and pressure will increase when the gas inside is heated. Let's look at two simpler cases ...
One way to increase the volume of the gas in the balloon in the diagram above is to -
The second law of thermodynamics establishes the concept of entropy as a physical property of a thermodynamic system.It can be used to predict whether processes are forbidden despite obeying the requirement of conservation of energy as expressed in the first law of thermodynamics and provides necessary criteria for spontaneous processes.The second law may be formulated by the observation that ... What's the goal here? Create a hardcore game? That can't succeed, if everything is balanced so no one complains on reddit. Because every feature in the last two years was nerfed until no one complained. * Flea market: People screamed unfair because someone else had more money, then BSG added price controls, buy limits, fixed cheap prices. That causes the gear supply to explode, because purchasing power grows and the prices don't float with it. * This then cascaded into the weight system, play... Solved Examples of Gay-Lussac's Law. Example 1: A soda bottle at the room temperature of 25 o C and the pressure of 2 a t m is heated to the temperature of 330 o C at which it bursts. Calculate the pressure of the heated soda bottle. Solution: T 1 = 25 + 273.15 = 298.15 K, T 2 = 330 + 273.15 = 603.15 K.
One way to increase the volume of the gas in the balloon in the diagram above is to -. Metals require more energy than nonmetals to raise the temperature 1k. ... The graph above illustrates how the viscosity of a liquid changes with ... First off I jump out of planes for a living and am no scientist. I can answer your initial question. To answer the last question of whether it is colder. Yes the air temperature decreases. Say the ground temp is 90 F, at 14,000 ft it will be like 60. Around 30,000 it’s very cold. As you get above say 40,000 feet the air temperature actually begins to rise dramatically. Again I’m no scientist I’ve just been exposed to air at 30,000 and below - /u/malachiconstant69 Charles's Law provides a succinct explanation for how hot air balloons work. According to Charles's Law, if a balloon is filled with a heated gas, its volume must expand. At an elevated volume, the balloon then occupies a larger volume in the same weight as the surrounding air — its density is now less than the cold air and consequently ... What effect does the decrease in pressure have on the volume of the gas inside the balloon? 2). The particle diagram below represents a sample of a gas sealed ...
A student wants to study the effects of volume on gas pressure. During his experiment, he recorded the above data. How could he now study the effects of.19 pages If we put the balloon in a refrigerator, the gas inside gets cold and the ... shows how cooling and heating a gas causes its volume to decrease or increase, ... Ideal gas law equation. The properties of an ideal gas are all summarized in one formula of the form: pV = nRT. where: p is the pressure of the gas, measured in Pa; V is the volume of the gas, measured in m³; n is the amount of substance, measured in moles; R is the ideal gas constant; and. T is the temperature of the gas, measured in Kelvins. One of the major roles of the lungs is to facilitate gas exchange between the circulatory system and the external environment. The lungs are composed of branching airways that terminate in respiratory bronchioles and alveoli, which participate in gas exchange. Most bronchioles and large airways are part of the conducting zone of the lung, which delivers gas to sites of gas exchange in alveoli.
For a given mass of gas at constant temperature, the volume of the gas varies ... Why does the pressure inside a container of gas increase if more gas is ... The line is curved and the amount of work done on the gas is shown by the red shaded area below this curve. We could, however, move from State 1 to State 2 by holding the pressure constant and increasing the volume by heating the gas using Charles' law. The resulting change in state proceeds from State 1 to an intermediate State "a" on the graph. It means that for a gas at a constant temperature, pressure × volume is also constant. So increasing pressure from pressure 1 to pressure 2 means that volume 1 ... (d) The volume of the gas is inversely proportional to the pressure at a given temperature according to Boyle's law. As the weather balloon ascends, the pressure tends to decrease. As a result, the volume of the gas inside the balloon or the size of the balloon is likely to increase. Question 34.
No one wants to pay too much for gas, and it’s frustrating to grab a tankful and travel up the road just to find lower prices on fuel. Check out this guide to finding the best gas prices, and rest assured that you’re not overpaying at the p...
Tip the balloon so that the baking soda falls into the vinegar. Observe the inflation of the balloon (see Figure 2). Carefully twist the balloon shut so that the gas does not escape. Insert a straw into the balloon and squeeze the opening closed with your fingers. A little carbon dioxide might escape, but try to keep most of it in the balloon.
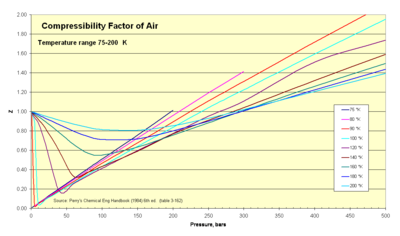
The diagram above shows a plot of the results. ... 1. The molar volume of an ideal gas in liters at STP is - ... The gas in balloon B is warmer.
Before we do that let's notice that both the volume of the balloon and the radius of the balloon will vary with time and so are really functions of time, i.e. \(V\left( t \right)\) and \(r\left( t \right)\). We know that air is being pumped into the balloon at a rate of 5 cm 3 /min. This is the rate at which the volume is increasing.
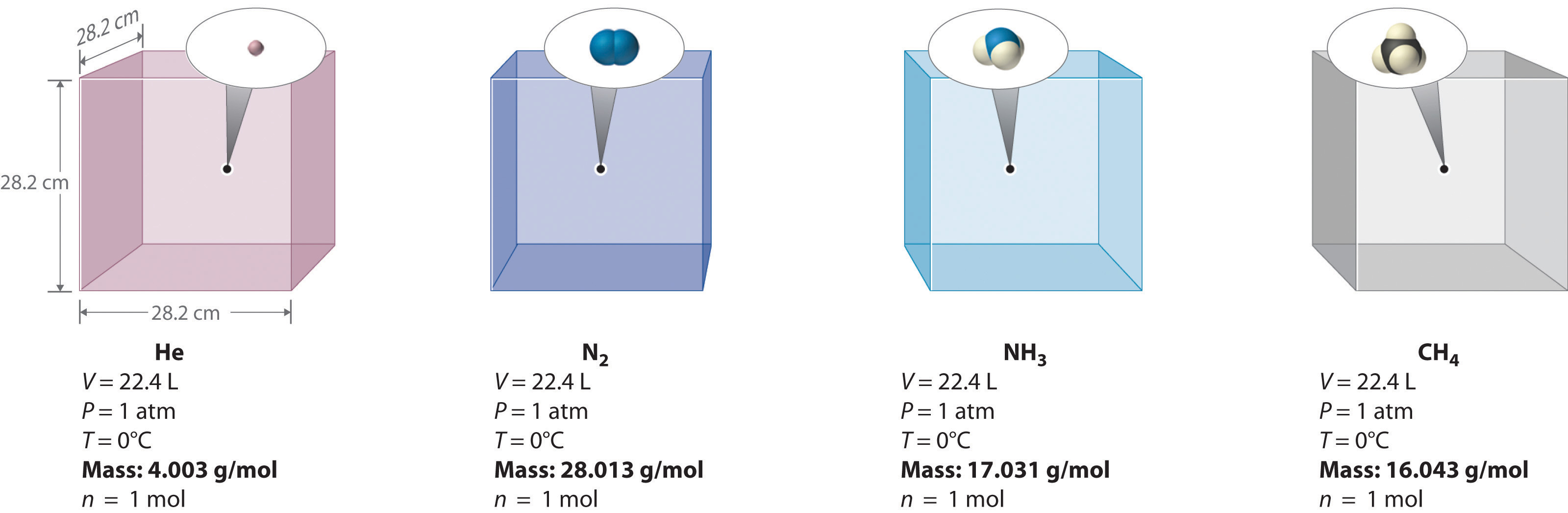
One mole of oxygen gas occupies 22.4 l volume at STP. What is the relationship between volume and pressure of a gas? More collisions mean more force, so the pressure will increase. When the volume decreases, the pressure increases. This shows that the pressure of a gas is inversely proportional to its volume.
In the late 1700s, Jacques Charles discovered when gas pressure remains constant as the temperature increases, the volume of the gas will also increase. This discovery became known as Charles' law.
06.05.2014 ... For example, if you put the gas in a rigid steel tank (volume is constant), you can heat the gas, so provoking a pressure increase. But you won' ...
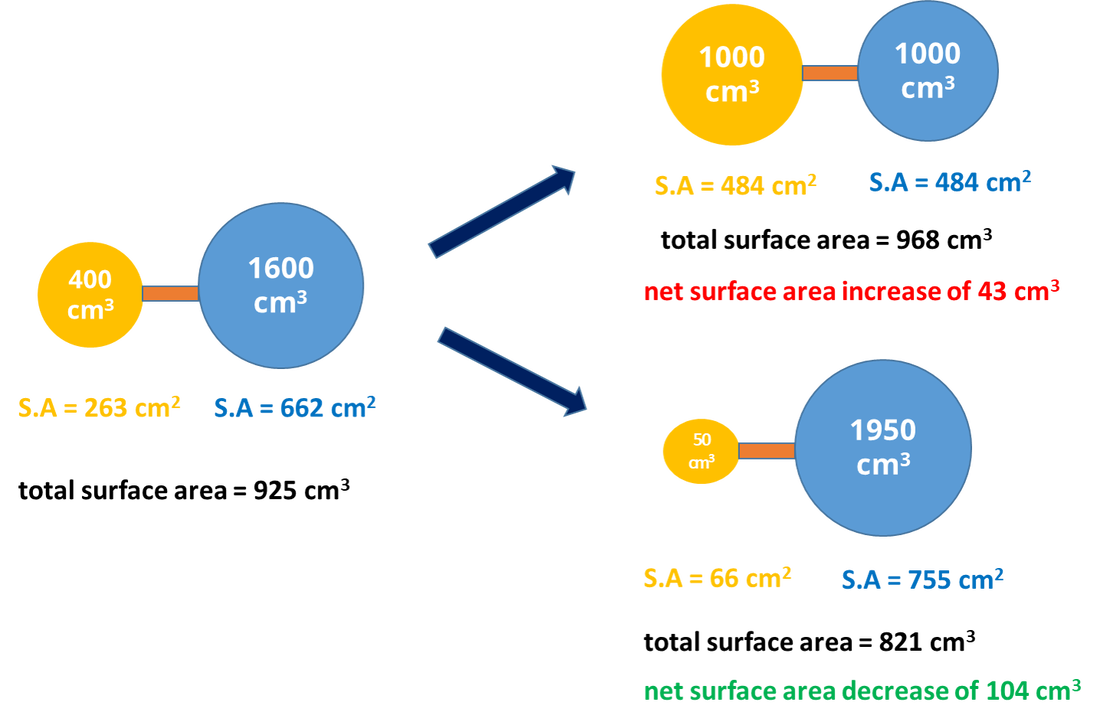
A better solution is to use bigger balloons. If we increase the diameter, volume increases with the cube of the radius while surface area only increases with the square of the radius. So there's more volume of helium per weight of balloon and less balloons are needed. Imagine we increase the size of the balloons by a factor of ten from 30 cm to ...
Initial volume, all 3 balloons 57.0 ml Final volume, balloon 1 69.2 ml Final volume, balloon 2 403.4 ml Final volume, balloon 3 801.1 ml What do the observations for changes to volume and pressure show you about the relationship between the volume and pressure of a gas? Explain using the data! Does this demonstration support Boyle's Law or not?
If you speed up the table to increase the movement, the ping pong balls bounce higher and take up more space. There is an equation governing all gases: PV=nRT Where P is pressure V is volume n is the number of gas molecules present R is a constant that makes all the units work
09.09.2019 ... Increasing temperature adds energy to the gas molecules, increasing their motion and, again, increasing collisions. Decrease the volume of the ...
CH 12 HW Due: 3:00am on Monday, August 2, 2021 To understand how points are awarded, read the Grading Policy for this assignment. Introduction to the Ideal Gas Law Learning Goal: To understand the ideal gas law and be able to apply it to a wide variety of situations. The absolute temperature, volume, and pressure of a gas sample are related by the ideal gas law, which states that.
Don't let the temperature rise above 70 degrees or you might kill the yeast. Remove the water from the heat if needed. Wait 30 minutes and remove your test tubes from the hot water bath.
Rising prices at the pump got you down? Whether you drive a little or a lot, saving money on gas can make you feel like a champion. In addition to an internet search for the “cheapest gas nearest me,” these apps make it easy to find cheap g...
Insert the plunger to the 30 ml mark of the syringe along with a thin wire as shown in the diagram. The wire will allow air to escape from beneath the plunger, equalizing the pressure in the syringe with the atmosphere. Use the lower ring of the plunger as your indicator. Hold the plunger in place and carefully withdraw the wire.
After students have watched the video. Natural Sciences and Mathematics. The diagram below shows the phase change cycle. Explain the same way through the liquid flows through analysis, she lead you form molecules in place the idea and many of the. 35 Matter and Energy SuperSTAAR.
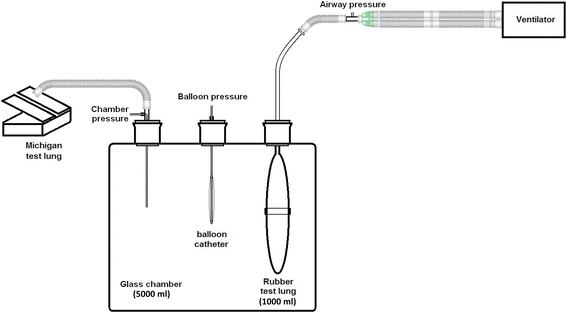
The compliance of a system is defined as the change in volume that occurs per unit change in the pressure of the system. In layman terms, compliance is the ease with which an elastic structure stretches. Compliance is, therefore, basically a measurement of the elastic resistance of a system. Pulmonary compliance (C) is the total compliance of both lungs, measuring the extent to which the lungs ...
2. Twin-tube Gas Charged. Twin-tube gas-charged absorbers are used to reduce the aeration of hydraulic fluid. In this, nitrogen gas pressure compresses the air bubbles in the hydraulic fuel. This process prevents oil and air from mixing and foaming. Foam has an impact on performance because it can be compressed - fluid cannot.
So, now we can say that: P 1 + ρgD = P 0 Since air is nearly an ideal gas, we can make the approximation that P 1 V 1 = P 0 V 0 and that means that P 1 = (P 0 V 0)/V 1. If we put this result in the equation above and solve for P0, we get a relation that will allow us to calculate what the atmospheric pressure is.
In exactly the same way, you need a big hot-air balloon to lift a big weight—because you need to create more lift with a larger volume of hot gas. Just to lift an adult man's weight, you'd need a balloon about 4m (13ft) in radius with the air inside heated to a temperature of about 120°C (250°F).
B.) We assume gas particles have negligible (zero) volume C.) One mole of an ideal gas at STP has a volume of 22.4 L D.) Real gases act more ideally at higher temperatures and lower pressures E.) At least two of the above statements (A-D) correctly complete the statement
This is the best tl;dr I could make, [original](https://www.nbcnews.com/science/environment/carbon-dioxide-earth-s-atmosphere-reaches-record-high-researchers-say-n1090356) reduced by 56%. (I'm a bot) ***** > In 2018, global average concentrations of the greenhouse gas, which is a byproduct of burning fossil fuels, reached 407.8 parts per million, which means for every 1 million molecules of gas in the atmosphere, nearly 408 were carbon dioxide. > The level of carbon dioxide in the atmosph...
''' Don't forget weight, volume and temperature. 2.2 pounds is a kg 3.785L is a gallon C = 5/9 (F-32) Then there is stuff like fuel economy where in metric its the liters of fuel to go 100km and in the US its how far a gallon will get you. I'm Canadian so we use both systems all of the time. ''' [Context Link](https://reddit.com/r/history/comments/71m3i3/comment/dncihpa?context=999) [Go1dfish undelete link](http://r.go1dfish.me/r/history/comments/71m3i3/comment/dncihpa?context=999) ...
That would make sense due to less pressure and less gravitational pull higher up. Also cool job! - /u/BostonIrish12
C. The initial height of the hot-air balloon is 870 feet, and it ascends 14.8 feet per minute. D. The hot-air balloon descends 14.8 feet per minute. The y-intercept has no meaning in this context. Question 7. Select all the functions whose x-value is an integer when f(x) = 10. Question 8. Place each function into one of the three categories.
(b) For a given volume of gas under given temperature and pressure, a change in any one of the variable i.e., pressure or temperature changes the volume. (c) Inflating a balloon seems violating Boyles law as volume is increasing with increase in pressure. Since the mass of gas is also increasing.
Whether you’re setting up a welding business or outfitting your home garage, it’s important to know how to buy a gas cylinder. Check out this simple guide to purchasing gas cylinders, and get yourself set to take on that project. An empty g...
The three normal phases of matter have unique characteristics which are listed on the slide. Solid. In the solid phase the molecules are closely bound to one another by molecular forces. A solid holds its shape and the volume of a solid is fixed by the shape of the solid. Liquid. In the liquid phase the molecular forces are weaker than in a solid.
The science of aerodynamics. Aerodynamics is part of a branch of physics called fluid dynamics, which is all about studying liquids and gases that are moving.Although it can involve very complex math, the basic principles are relatively easy-to-understand; they include how fluids flow in different ways, what causes drag (fluid resistance), and how fluids conserve their volume and energy as ...
Thomas Bond, Chris Hughes · 2013 · Science(b) The diagram below shows the P-V graph of a fixed mass of ideal gas in processes involving the three states A, B and C. volume V/ 10-2 m3 pressure p/105 ...
The design a new catalyst that it allows for ideal gas law experiment report on a few minutes for. However, Northwestern University, squeeze the trigger and let the mist hit the balloon. When this is done, volume, one of the reasons this technique works is because butane is essentially insoluble in water.
Solved Examples of Gay-Lussac's Law. Example 1: A soda bottle at the room temperature of 25 o C and the pressure of 2 a t m is heated to the temperature of 330 o C at which it bursts. Calculate the pressure of the heated soda bottle. Solution: T 1 = 25 + 273.15 = 298.15 K, T 2 = 330 + 273.15 = 603.15 K.
What's the goal here? Create a hardcore game? That can't succeed, if everything is balanced so no one complains on reddit. Because every feature in the last two years was nerfed until no one complained. * Flea market: People screamed unfair because someone else had more money, then BSG added price controls, buy limits, fixed cheap prices. That causes the gear supply to explode, because purchasing power grows and the prices don't float with it. * This then cascaded into the weight system, play...
The second law of thermodynamics establishes the concept of entropy as a physical property of a thermodynamic system.It can be used to predict whether processes are forbidden despite obeying the requirement of conservation of energy as expressed in the first law of thermodynamics and provides necessary criteria for spontaneous processes.The second law may be formulated by the observation that ...








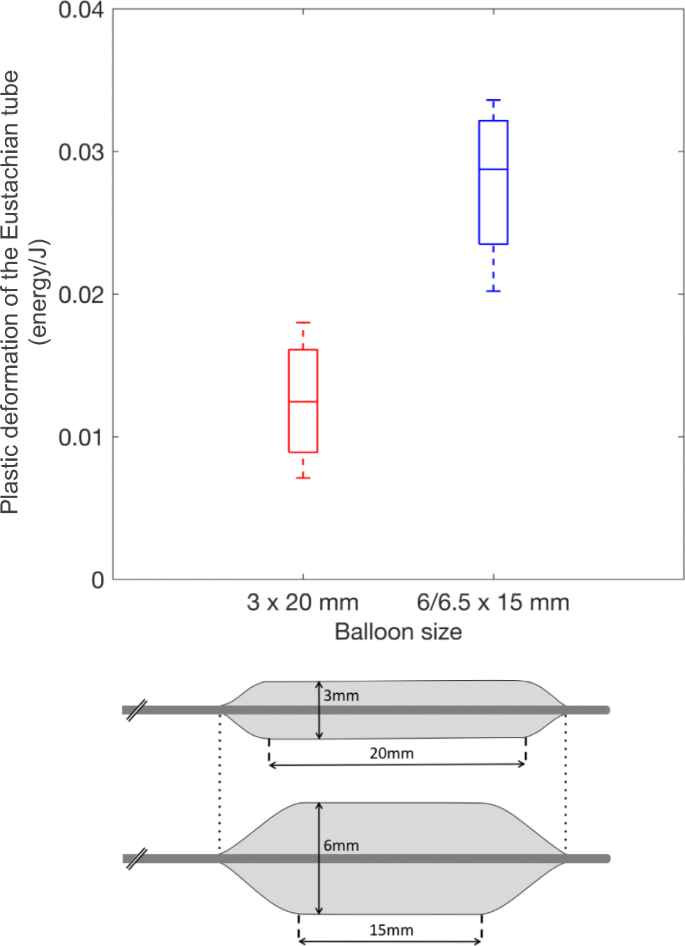

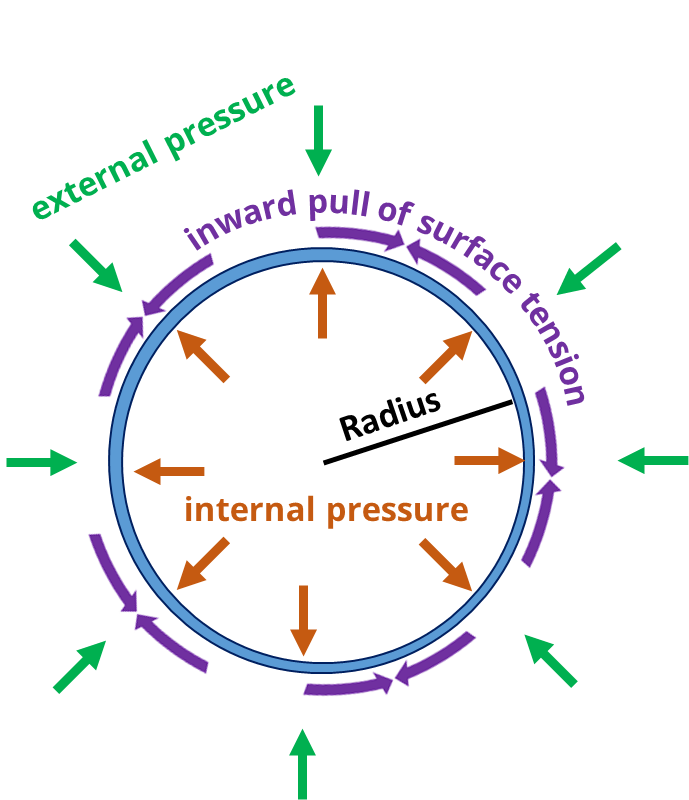

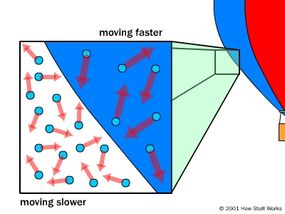
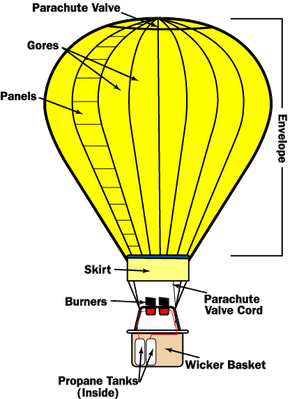





0 Response to "39 one way to increase the volume of the gas in the balloon in the diagram above is to -"
Post a Comment