40 energy level diagram for helium
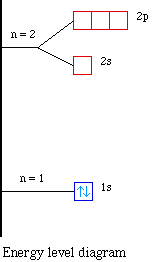
A diagram shows an He atom with two electrons in its 1s orbital and an He+ Energy-level diagram for the He2+ ion. Which electrons in this diagram ...Sep 17, 20201 answer · Top answer: 1/2 Live. •. Helium only has 2 electrons and therefore it has a configuration of 1s 2. Because the 1s orbital is full with 2 electrons and any additional electrons would go in a new energy level. The electron configuration for Helium shows a full outer shell and is Helium is therefore called a Nobel Gas. This means it will not react with other atoms.
The helium atom. 1. Draw an energy level diagram showing all helium excited states. Label the energy levels… according to. (a) Configurations.24 pages

Energy level diagram for helium
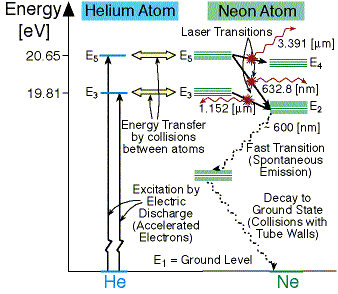
The energy level diagram for a helium ion (He+) is shown below. How much energy is required to remove the remaining electron from the helium ion? You may assume that Het is initially in its ground state. He+ Ionisation Levels 0 -0.85 -1.11 -1.51 -2.18 -3.40 -6.04 Energy (en) -13.6 -54.4 Ground State Select one: O a. O eV O b. +54.4 eV. O c. +13 ... I'm coming from a Physics background and am trying to make sense of the energy level diagram for para-helium and ortho-helium. From what I've gathered each column shows the different energy levels for different orbitals so that for example the leftmost column with $^1S$ written on top shows the energy levels of 1s, 2s, 3s etc. Helium Neon Laser Energy Level Diagram. Here upward transition shows the absorption of energy from the pumping source by Helium atom. While down ward transition shows the emission of energy / light or lasing present in the Neon atom only. In diagram above there are 3 down word energy transitions for Neon that produce lasing.
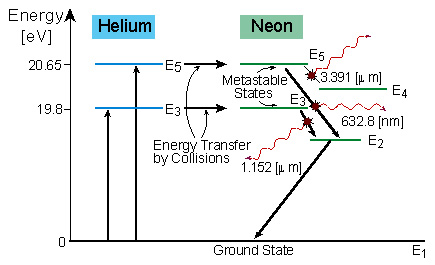
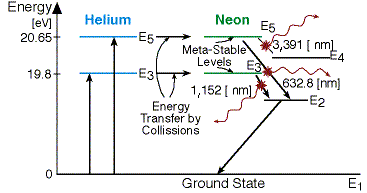
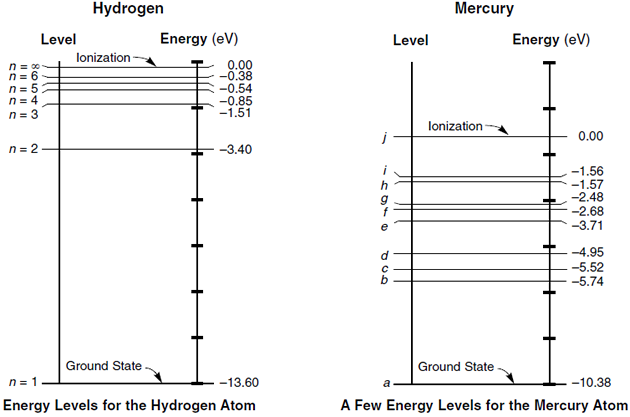
Energy level diagram for helium. The energy level diagram for He and Ne atoms are shown in Fig2. When an electric discharge passes through the gas, the electron in the discharge tube collide with the He and Ne atoms and excite them to metastable states of energy 20.61 eV and 20.66 eV respectively above the ground state. Some of the excited helium atoms transfer their energy to ... And see the energy level for He I (neutral He) and H I (neutral Hydrogen) in the unit of eV. I discover something: The energy level of Helium is just energy level of Hydrogen plus 10 eV. My hypothesis is that this extra energy comes from the repulsion energy between the 2 electrons of Helium when one in excited state and one in ground state. Forms of such diagrams are called Grotrian diagrams or term diagrams in various parts of the literature. While the energy level diagram of hydrogen with its single electron is straightforward, things become much more complicated with multi-electron atoms because of the interactions of the electrons with each other. The Bohr model of Helium is drawn with only one electron shell and it contains 2 electrons. Helium is neutral and its atomic number is 2, hence, the number of protons and electrons available for its Bohr diagram is also 2. The number of neutrons for the Bohr diagram of Helium can be found by subtracting the number of protons from the atomic ...
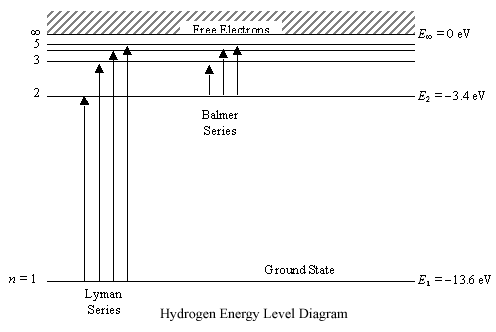
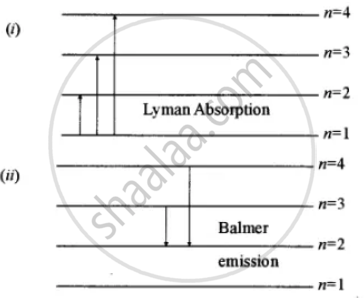
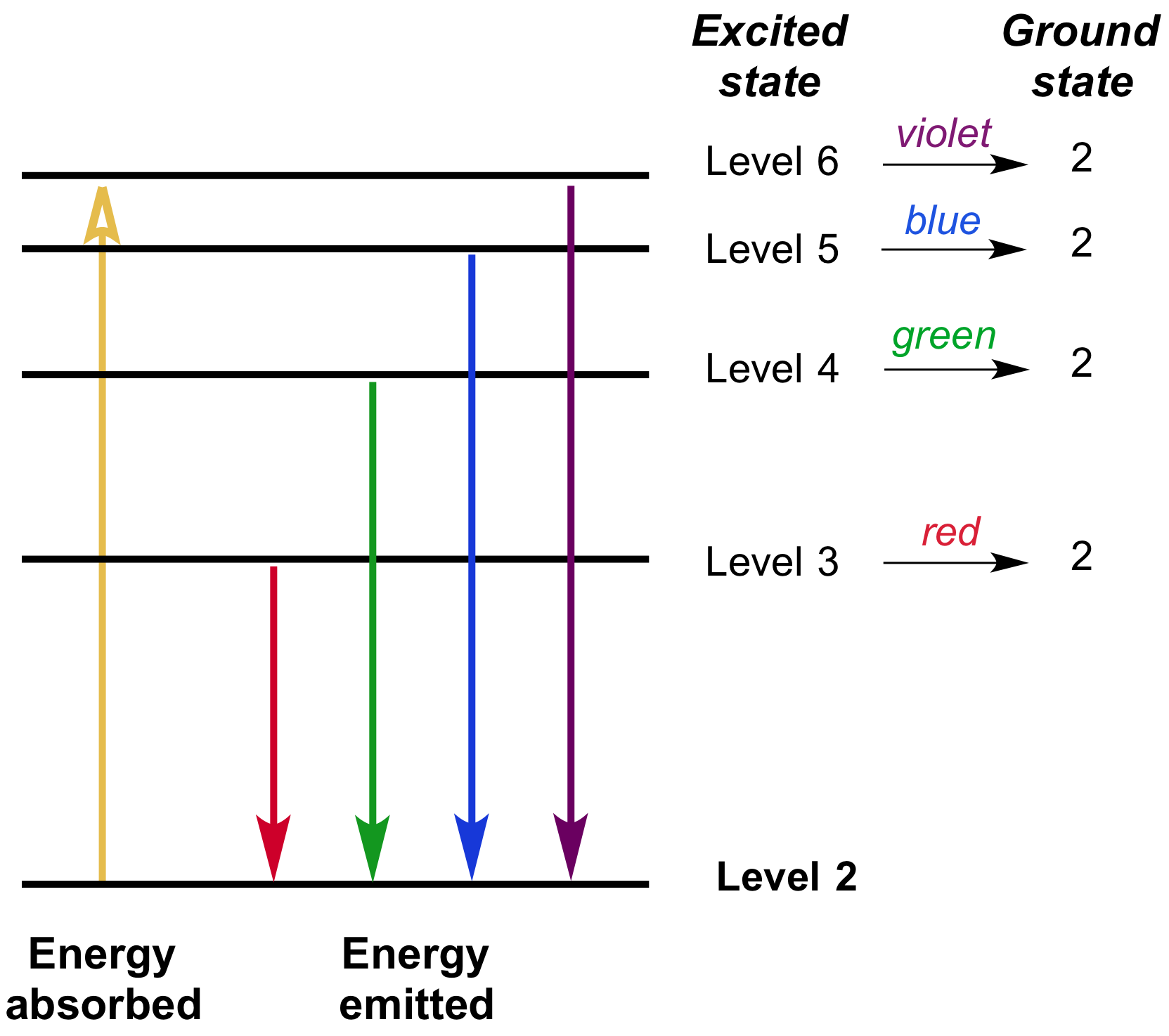
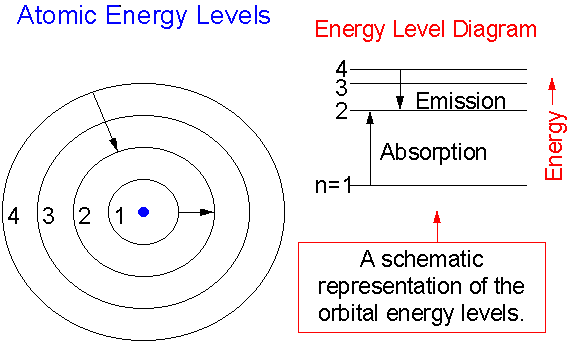
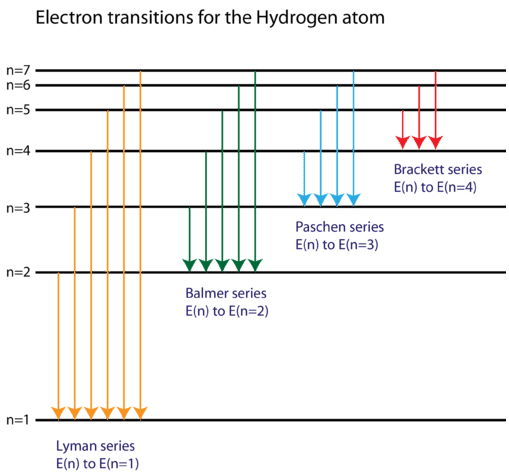
PhysicsLAB: Energy-Level Diagrams. Energy level diagrams are a means of analyzing the energies electrons can accept and release as they transition from one accepted orbital to another. These energies differences correspond to the wavelengths of light in the discreet spectral lines emitted by an atom as it goes through de-excitation or by the ... Orthohelium and Parahelium Energy Levels In the helium energy level diagram, one electron is presumed to be in the ground state of a helium atom, the 1s state.An electron in an upper state can have spin antiparallel to the ground state electron (S=0, singlet state, parahelium) or parallel to the ground state electron (S=1, triplet state, orthohelium). between the E3 and E1 energy levels of an ionized helium atom. 8. Indium was named for the bright indigo line in its emission spectrum. This line is produced when an electron drops from the E2 energy level to the E1 energy level (depicted in the diagram below). Use the diagram to calculate the energy of this emitted photon, then calculate the ... An energy level diagram looks like a ladder with the allowed energies represented as horizontal lines, and the energies increasing as you go up. It's a good way ...6 pages
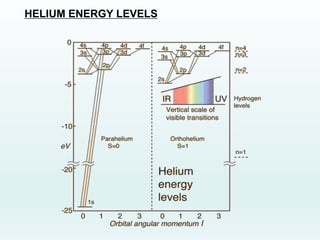
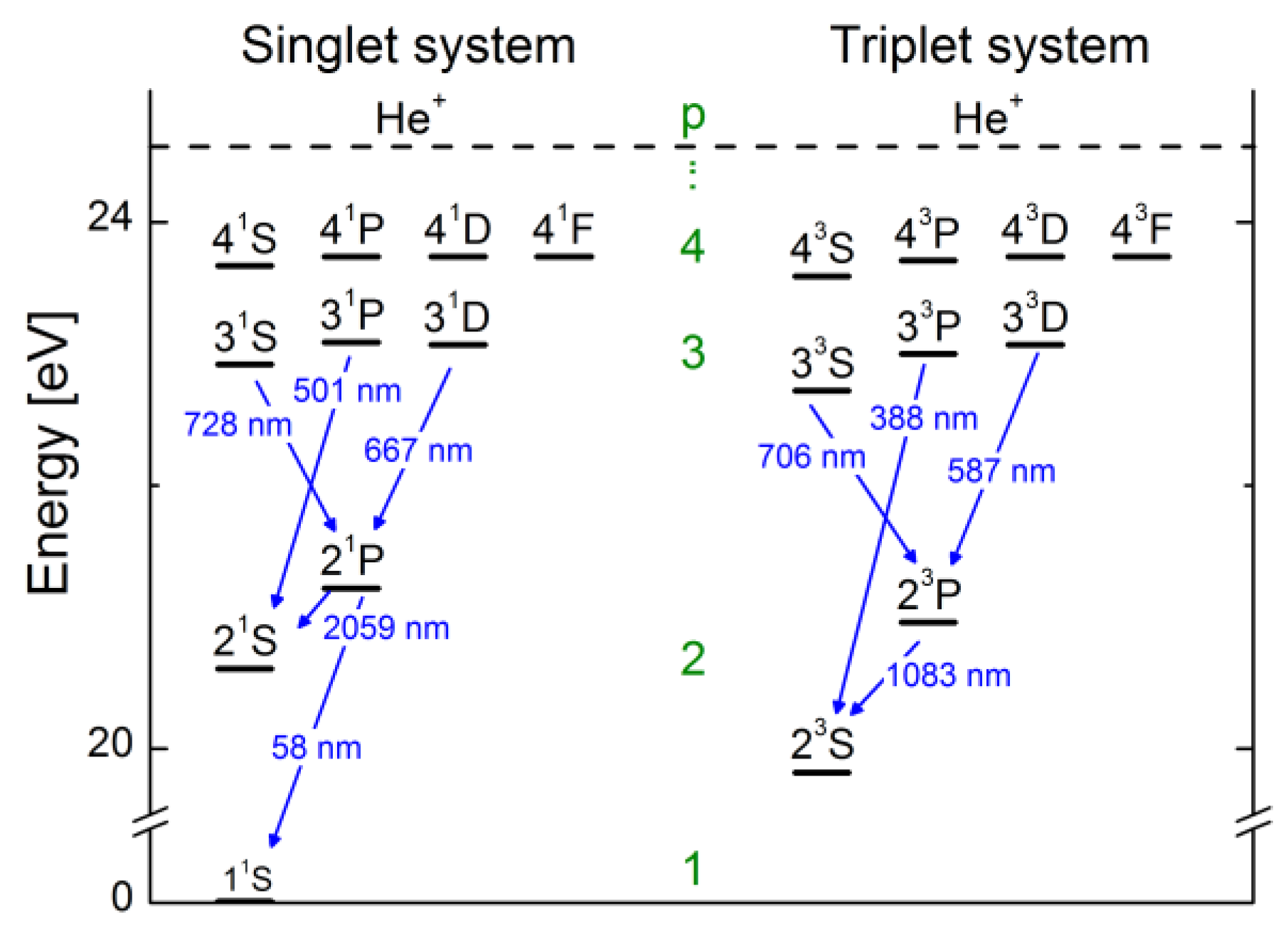
Energy Levels of Helium Introduction In this experiment you will verify the quantization of energy levels in the helium atom, and measure the energy levels, using a critical potential tube. All electrons within atoms are restricted by quantum theory to discrete energy levels as originally predicted by Bohr's model of the atom. Helium energy level diagram Helium ground state 1s 2 1S0 Helium excited states 1snl 1LJ or 3LJ. He 1s2p fine structure, compared to 1s2s level position Units: due to variety of energy scales in atomic physics, you may see energies displayed in Orthohelium, with a multiplicity of 3 has a lower energy than parahelium, with a multiplicitiy of 1. Conclusions. In this quantitative investigation, the total singlet-triplet splitting of the 2p valence electrons of helium was seen to be 0.22 eV + 0.03 eV = 0.25 eV, as is shown in the energy level diagram displayed earlier. An energy level diagram for helium is shown in Figure 1. The singlet states are on the left and the triplet states are on the right. Note that "singlet" states are those in which the two helium electrons have opposite spin, while "triplet" states are those whose electrons have the same spin. Figure 1.
Construct the energy-level diagram (like Fig. 37-26) for (a) He+ ion and (b) doubly ionized lithium Li2+. Solution 9.3. (a) Singly ionized helium is like ...4 pages
Energy level diagram of Helium Neon laser » LASER. Working of Helium Neon Laser. In the earlier articles, I have explained the construction of helium neon laser. Today let us discuss the working of helium neon laser: Pumping of. by amsh 3 Min Reading. RECEIVE ARTICLES BY EMAIL: Enter your email address:
Figure 4 depicts, as a representative case, the level diagram of parahelium. The helium states and energy levels can be classified as follows: (i) the ground state and bound singly excited states ...
Helium (He) Energy Levels of Neutral Helium ( He I ) Configuration : Term : J : Level(cm-1): Ref. 1s 2: 1 S: 0: 0.000: M02 : 1s2s: 3 S: 1
Helium only has 2 electrons and therefore it has a configuration of 1s2. Because the 1s orbital is full with 2 electrons and any additional electrons would go in a new energy level. The electron configuration for Helium shows a full outer shell and is Helium is therefore called a Nobel Gas.
Working of He-Ne. It is a four energy level laser system. The electrons produced from electric discharge collide with He and Ne atom and excite them to the higher energy levels He2 and Ne4 at 20.61 eV and 20.66 eV respectively. These two states are metastable so the atoms may stay there for a longer time. They are very close to each other, thus ...
The Helium-Neon laser was the most common laser until the spread of diode lasers in the last few years. It was first built in 1961 by Ali Javan. The active medium is a noble gas Neon (Ne), and it is a 4 level laser. The energy level diagram of a Helium-Neon laser is described in figure 6.1. Two meta-stable energy levels act as upper laser levels.
Download scientific diagram | Energy-level diagram of pertinent helium singlet states involved in observed photons and important cascade routes. from publication: Electron-impact excitation of the ...
ground state and the two lowest excited states of helium. To put these results into context, please look at the energy level diagram in Section 5.2.1 of Gri ths. This truncated-matrix approach to the helium atom, including the Mathematica code that I'll show in class, is based on a recent article by Robert C. Mass e and
The energy level diagram of helium and neon atom is shown below. he ne laser energy level diagram. energy level diagram of He-Ne laser. When electric discharge occurs in the discharge tube, some of the atoms of the mixture of He-Ne that ionized. The electrons obtained so collide with Helium atom in the ground state or level and hence Helium ...
Millions of thanks from depths of My Heart to every subscriber and Viewer.Fun Food Fashion Fitness with ""S4F"' (my Second Channel)https://www.youtube.com/ch...
Figure 3.4 show the energy level diagram of Helium-Neon laser, with the possible transitions. The mass of the Helium atom is about one-fifth of the mass of the Neon atom. The amount of Helium in the tube is about 6 times the amount of Neon. Thus Helium atoms have more chance to receive energy from the accelerated electrons, and transfer into ...
Helium Neon Laser Energy Level Diagram. Here upward transition shows the absorption of energy from the pumping source by Helium atom. While down ward transition shows the emission of energy / light or lasing present in the Neon atom only. In diagram above there are 3 down word energy transitions for Neon that produce lasing.
I'm coming from a Physics background and am trying to make sense of the energy level diagram for para-helium and ortho-helium. From what I've gathered each column shows the different energy levels for different orbitals so that for example the leftmost column with $^1S$ written on top shows the energy levels of 1s, 2s, 3s etc.
The energy level diagram for a helium ion (He+) is shown below. How much energy is required to remove the remaining electron from the helium ion? You may assume that Het is initially in its ground state. He+ Ionisation Levels 0 -0.85 -1.11 -1.51 -2.18 -3.40 -6.04 Energy (en) -13.6 -54.4 Ground State Select one: O a. O eV O b. +54.4 eV. O c. +13 ...




















0 Response to "40 energy level diagram for helium"
Post a Comment