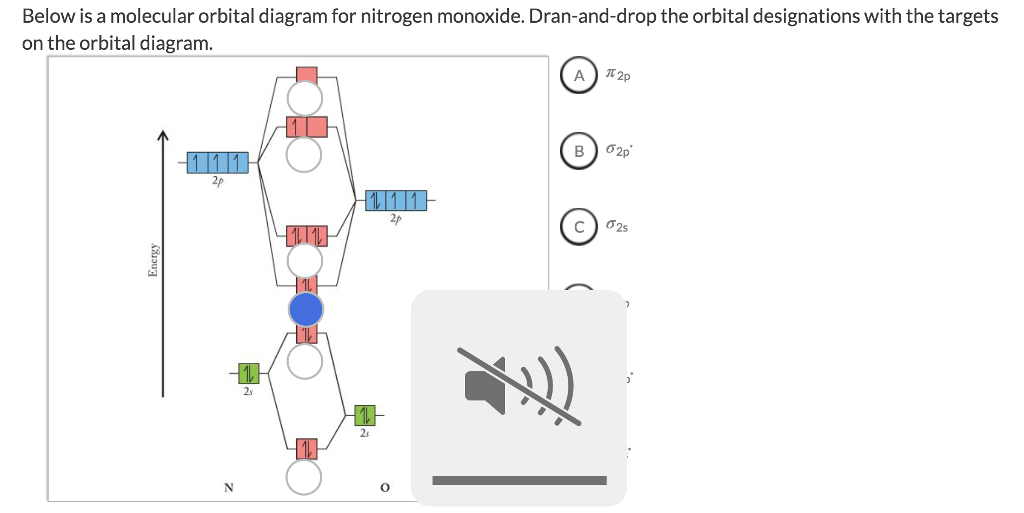
40 nitrogen molecular orbital diagram
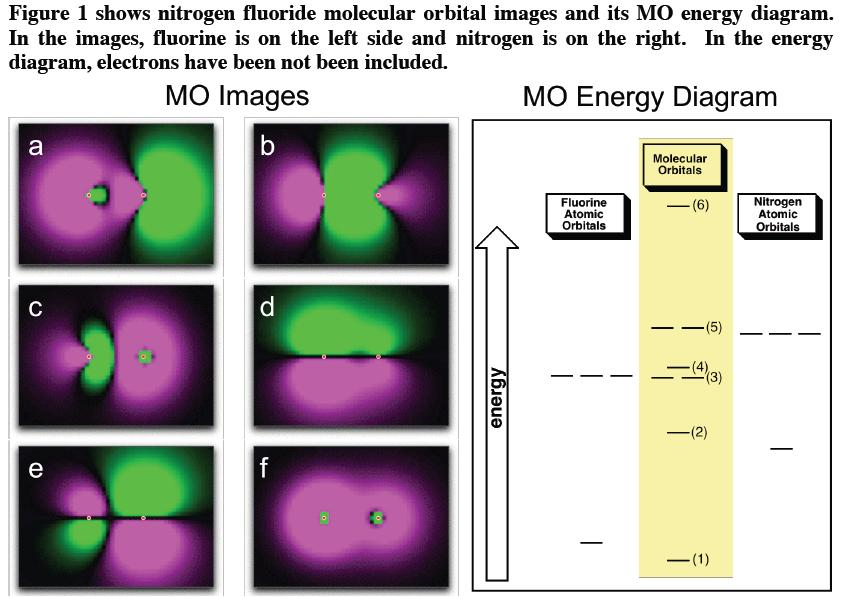
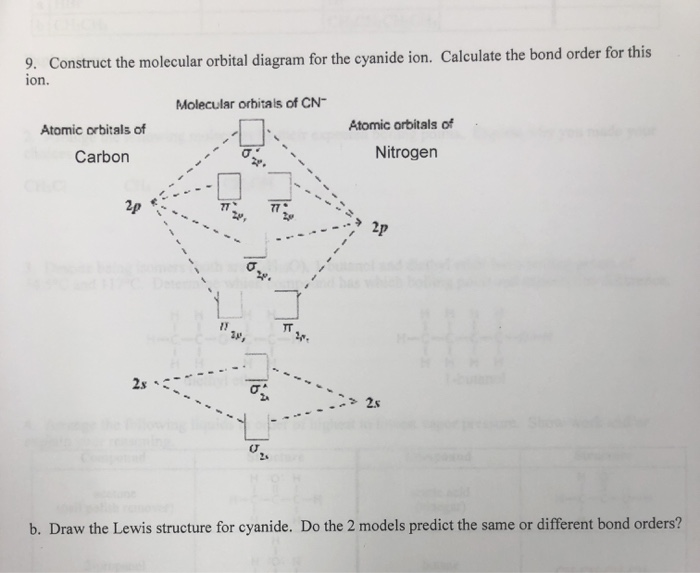
A molecular orbital model for 1,3-butadiene is shown below. Note that the lobes of the four p-orbital components in each pi-orbital are colored differently and carry a plus or minus sign. This distinction refers to different phases, defined by the mathematical wave equations for such orbitals. Jan 04, 2022 · Now the next topic to cover is the molecular orbital diagram of nitrous oxide. N2O Molecular Orbital Diagram. Molecular orbital diagrams say about the mixing of orbitals in a compound. Using a MO diagram, the bond order of a compound can be determined which gives us an idea about bond length, bond stability as well.
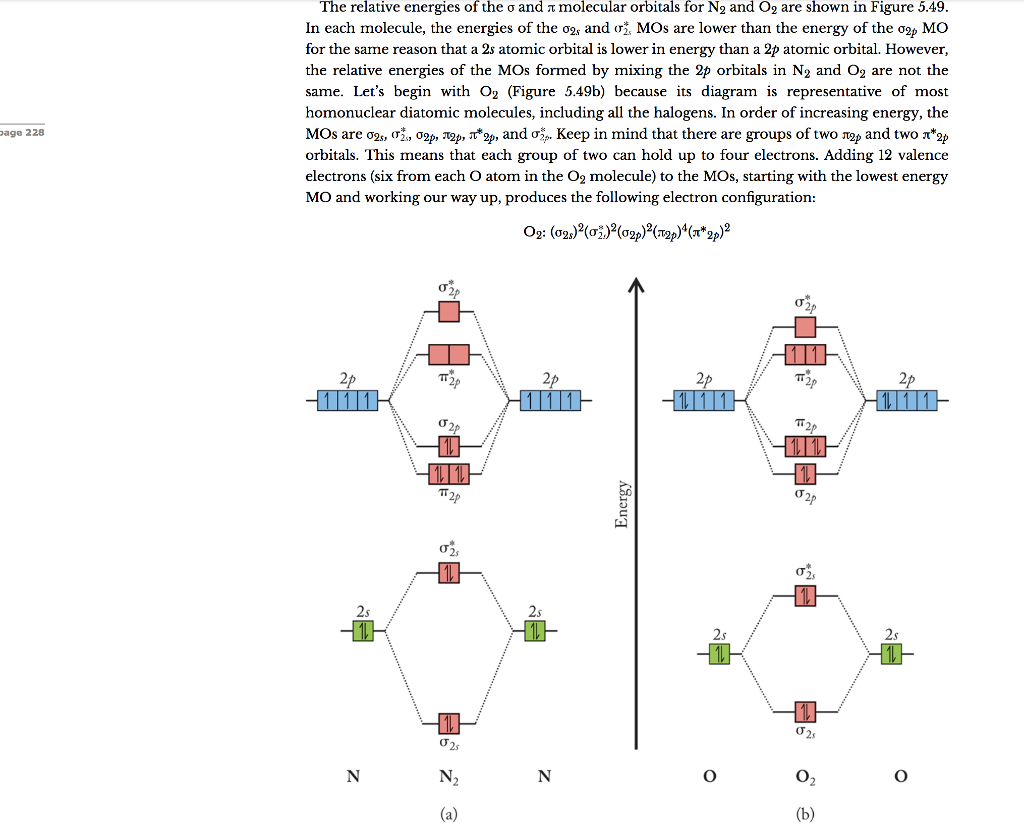
O2 and N2 have different number of electrons. Nitrogen is in the fifth group and oxygen in the sixth group of the periodic table. Nitrogen has 7 electrons and ...3 answers · Top answer: Here is the MO diagram for O₂: Whilst this is the MO diagram for N₂: If we compare ...
Nitrogen molecular orbital diagram
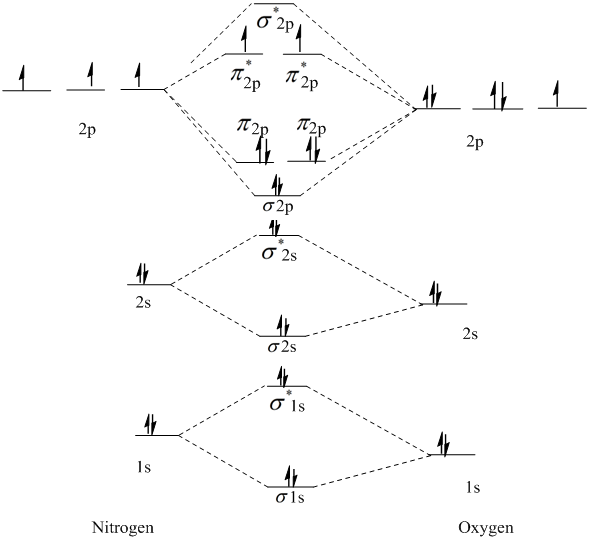
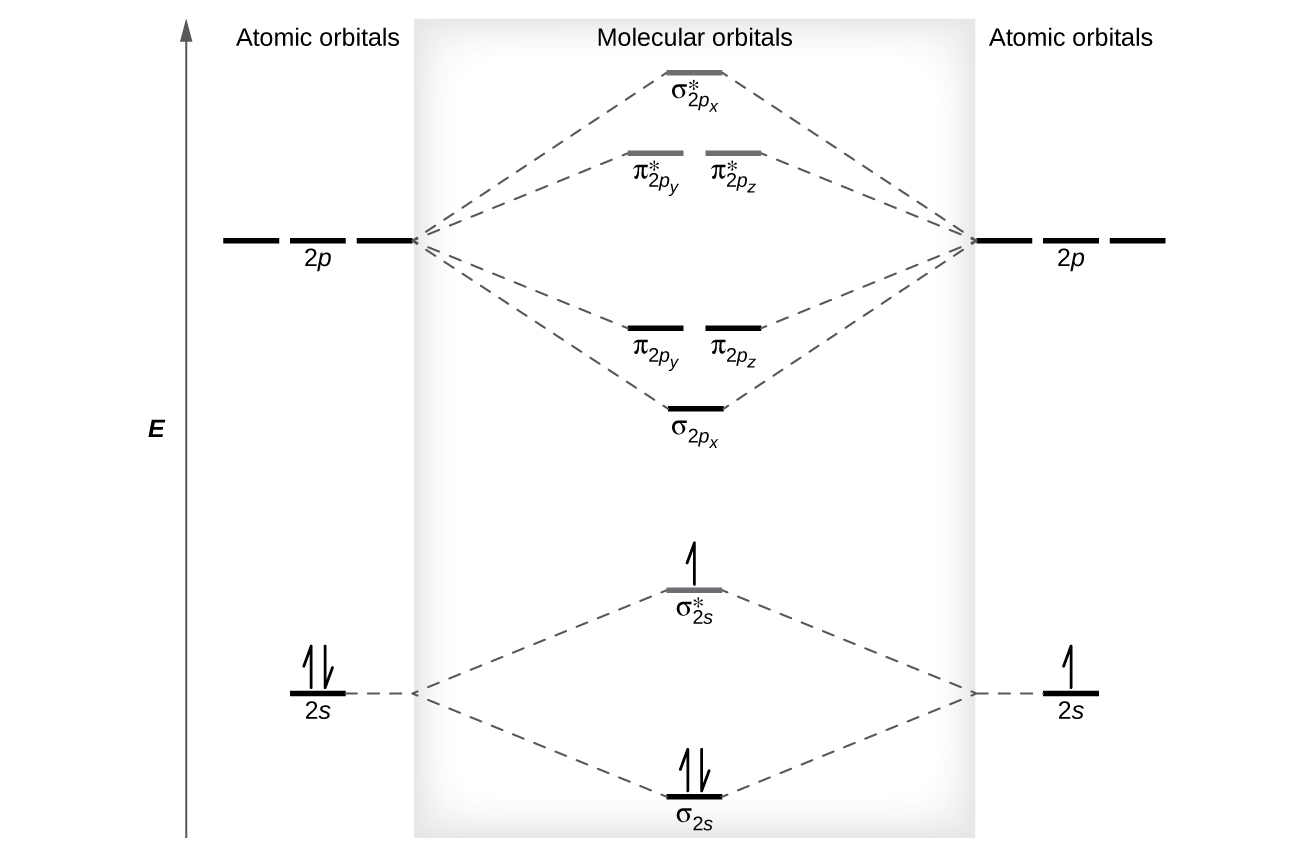
Mar 19, 2021 · A quantitative molecular orbital calculation with a computer would not take this shortcut, but would include all of the electrons in the atoms that are bonding together. Nitrogen has five valence electrons, and these electrons are found in the 2s and 2p levels. 26 Nov 2018 — In nitrogen MO , there is a slight change in MO diagram when compared to the MO diagrams of O2 , F2 molecules.Here,the σ2p orbital is higher in ... Atomic nitrogen has 5 valence electrons and 4 valence orbitals (2s, 2px, 2py, and 2pz). In the Lewis structure there is a triple bond between the nitrogen ...
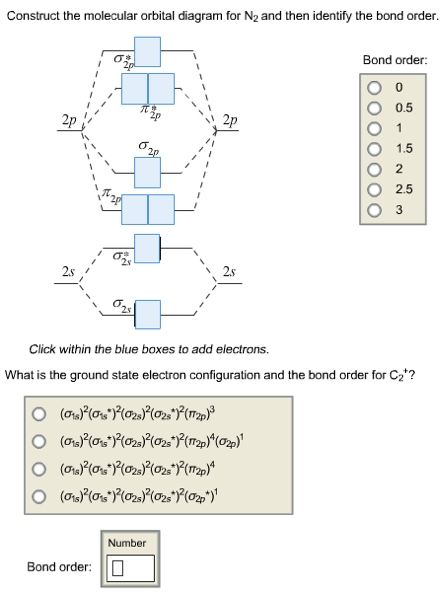
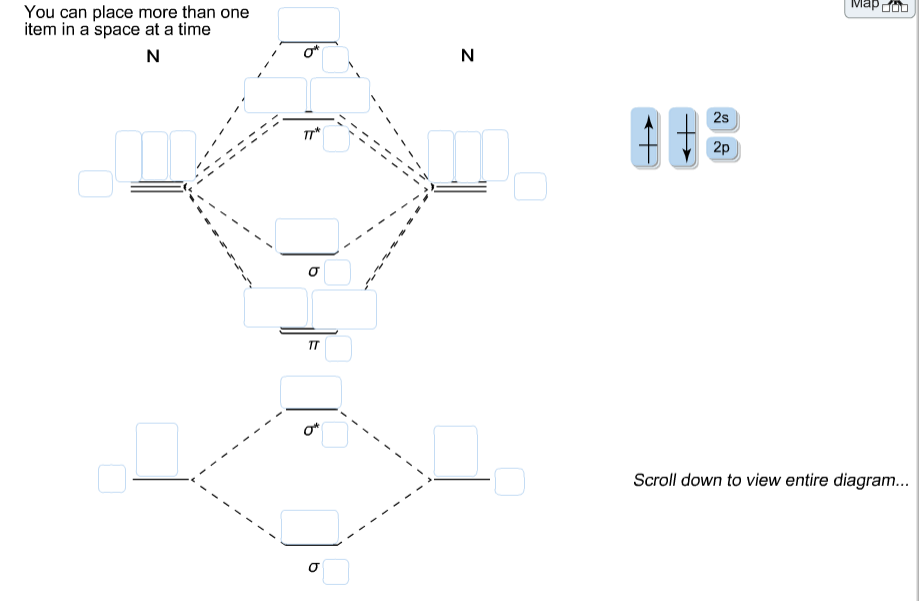
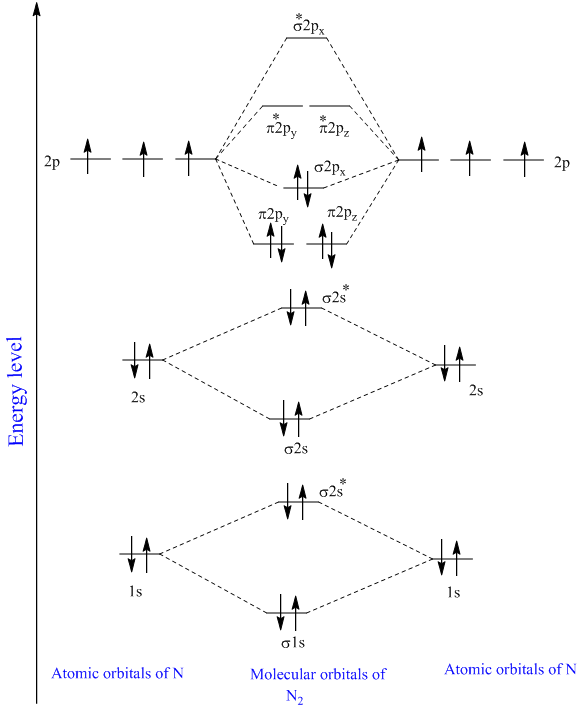
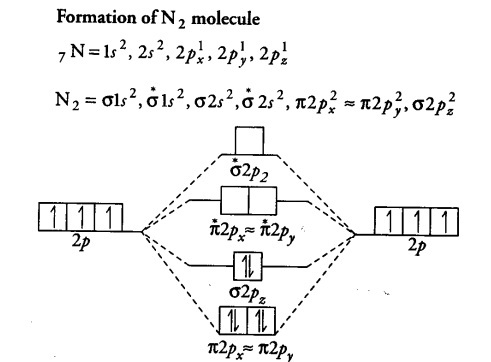
Nitrogen molecular orbital diagram. With nitrogen, we see the two molecular orbitals mixing and the energy repulsion. This is the reasoning for the rearrangement from a more familiar diagram. Each nitrogen has five valence electrons, for a total of ten, so we have just filled in ten electrons, starting at the bottom of the molecular orbital energy level diagram. If this were another molecule, such as F 2 or O 2 , we would construct the overall diagram in a similar way, but just use a different number of electrons. Draw the molecular orbital diagram of N2. Also find its bond order and magnetic · Asked by Topperlearning User | 13th Jun, 2016, 02:45: PM. Expert Answer: ... Electrons of nitrogen are to be filled in this diagram. Left side represents the configuration of one atom of nitrogen molecule and the right side represents ...
Each nitrogen atom has the electron configuration 1s22s22p3. These atomic electrons are shown on either side of the molecular orbital diagram. Atomic nitrogen has 5 valence electrons and 4 valence orbitals (2s, 2px, 2py, and 2pz). In the Lewis structure there is a triple bond between the nitrogen ... 26 Nov 2018 — In nitrogen MO , there is a slight change in MO diagram when compared to the MO diagrams of O2 , F2 molecules.Here,the σ2p orbital is higher in ... Mar 19, 2021 · A quantitative molecular orbital calculation with a computer would not take this shortcut, but would include all of the electrons in the atoms that are bonding together. Nitrogen has five valence electrons, and these electrons are found in the 2s and 2p levels.















.png)


![[DIAGRAM] Molecular Orbital Diagram Of Ammonia FULL ...](https://cdn1.byjus.com/wp-content/uploads/2019/08/orbital-diagram-of-nitrogen.png)

0 Response to "40 nitrogen molecular orbital diagram"
Post a Comment