41 b2 2- molecular orbital diagram
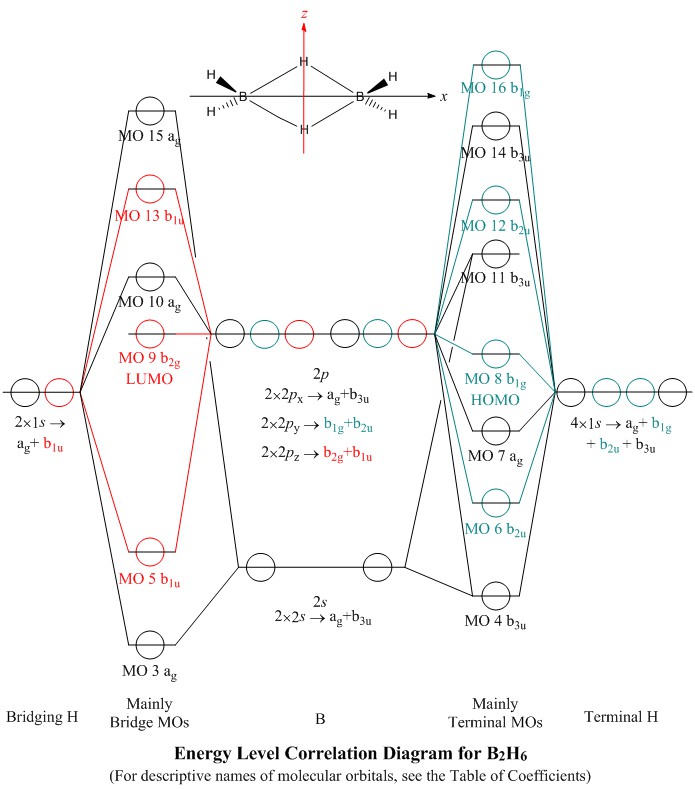
Nov 11, · As discussed in class the MO diagram for B 2 shows that it has two unpaired electrons (which makes it paramagnetic) and these electrons are in bonding molecular orbitals resulting in the equivalent bond strength of one bond. As discussed in class it is not a bond. 2 linear. BH2. 2a1. 21b2. 23a1. MO Diagram for BeH2. Be 2p. Be 2s. –. +. BeH2 MOs bonding MOs antibonding MOs non-bonding orbitals. Be AOs. H LCAOs g u u bonding nonrbonding. a complex MO diagram: B2H6 MO diagrams combine two fragments. Symmetry .. fragment =5e therefor keep up to b2 orbital z x y b1 a1 a1 b2. BH2. Fig.
The molecular orbitals having the same sign combine and give bonding molecular orbitals. We have to draw the molecular orbital diagram for ${{\text{B}}_{\text{2} ...
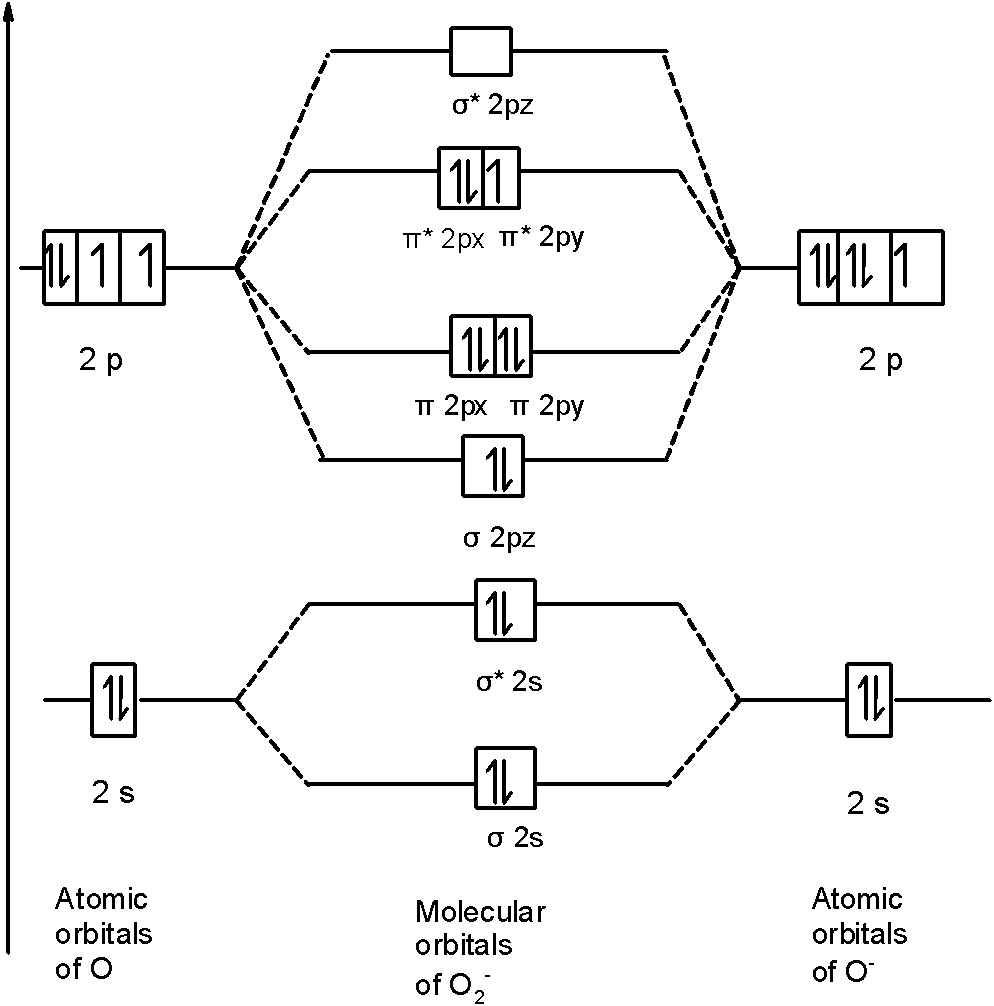
B2 2- molecular orbital diagram
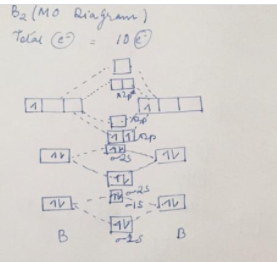
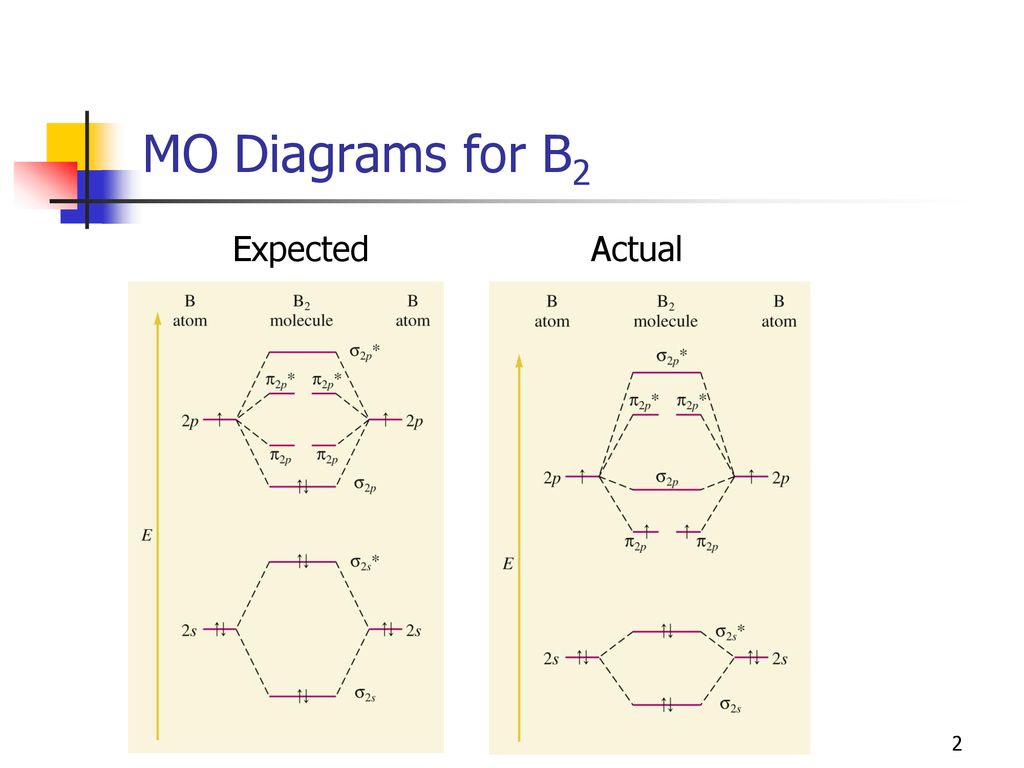
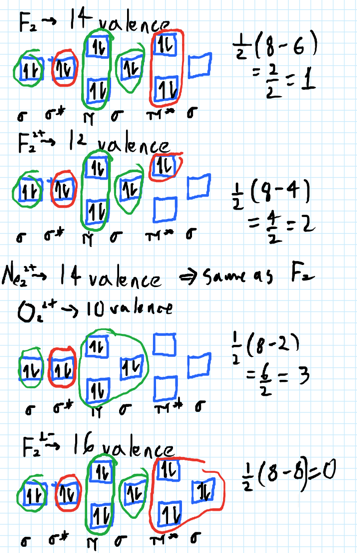
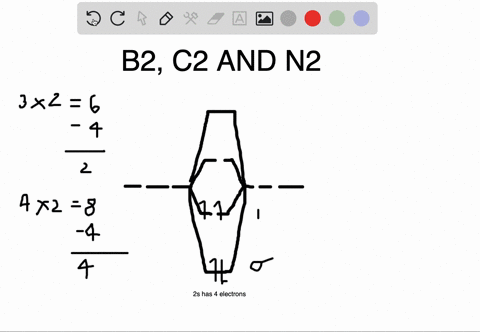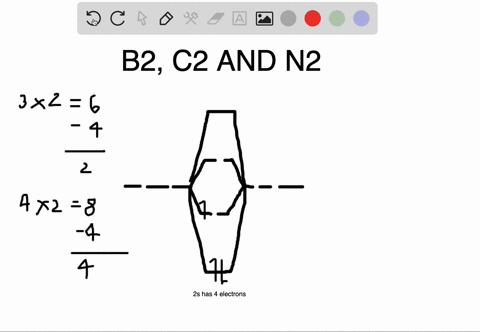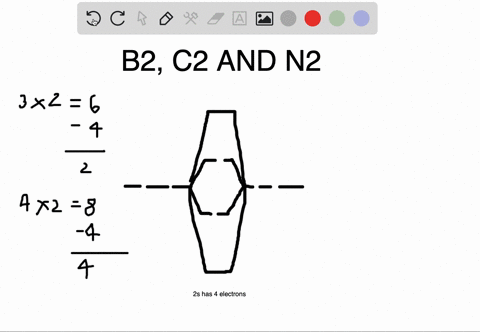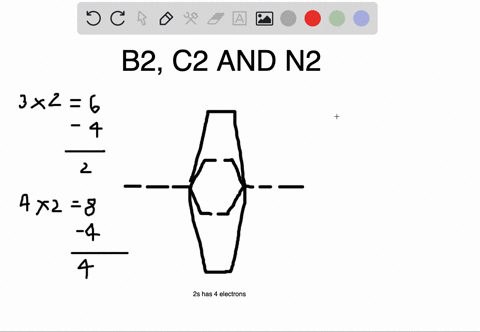
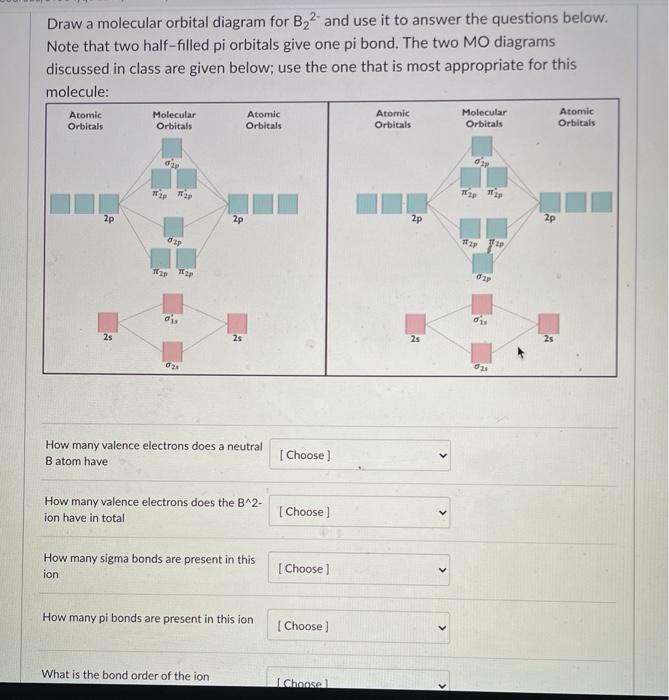
The number of unpaired electrons in the B2+ molecule is 2 (two) 1 (one) 3 (three) zero ; Question: Draw the molecular orbital diagram for B2+ (this is not B-B. it has an electron missing, so its B-B cation!) The number of unpaired electrons in the B2+ molecule is 2 (two) 1 (one) 3 (three) zero When you write the M.O. Diagram for B2, this is what you get: This shows two unpaired electrons in π2px and π2pz. However, the Bond Order of B2 = 1/2 (4-2) = 1. Doesn't this mean that B2 has a single bond between the two Boron atoms--therefore they have one sigma bond? The molecular orbital electronic configuration, Magnetic property: Since bond order is zero, Be 2 molecule does not exist. It is diamagnetic due to the absence of any unpaired electron. B 2 molecule: The electronic configuration of B atom (Z = 5) is. B 2 molecule is formed by the overlap of atomic orbitals of both boron atoms. A number of ...
B2 2- molecular orbital diagram. Bh2 molecular orbital diagram. a) Construct a molecular orbital energy-level diagram for BH 2 + H s B s p (Orbital Potential Energies: , 1 : -13.61 eV; : 2 , -14.05 eV, 2 : -8.30 eV) b) Sketch the molecular orbital pictures (schematic representation) for the HOMO and LUMO of BH 2 + . When two fluorine atoms bond, the sigma(2p) bonding molecular orbitals are lower in energy than the pi(2p) bonding orbitals.F2(2+) has a bond order of 2, so ... B2 Molecular Orbital Diagram. Collected from the entire web and summarized to include only the most important parts of it. Can be used as content for research and analysis. Home Blog Pro Plans Scholar Login. Advanced searches left . 3/3. Search only database of 12 mil and more summaries ... Jan 27, 2015 Before we can draw a molecular orbital diagram for B₂, we must find the in-phase and out-of-phase overlap combinations for boron's atomic orbitals. Then we rank them in order of increasing energy. We can ignore the 1s orbitals, because they do not contain the valence electrons. Each boron atom has one 2s and three 2p valence orbitals.
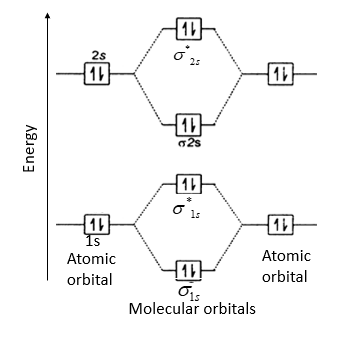
Molecular Orbital Theory allows us to predict the distribution of electrons within a molecule. This allows us to predict properties such as bond order, magnetism, and shape.. There are two types of MO diagrams:. Recall that the bonding MOs are those without an asterisk (e.g., σ 1s), while the antibonding MOs are those with an asterisk (e.g., σ 1s *). The molecular orbital electronic configuration, Magnetic property: Since bond order is zero, Be 2 molecule does not exist. It is diamagnetic due to the absence of any unpaired electron. B 2 molecule: The electronic configuration of B atom (Z = 5) is. B 2 molecule is formed by the overlap of atomic orbitals of both boron atoms. A number of ... When you write the M.O. Diagram for B2, this is what you get: This shows two unpaired electrons in π2px and π2pz. However, the Bond Order of B2 = 1/2 (4-2) = 1. Doesn't this mean that B2 has a single bond between the two Boron atoms--therefore they have one sigma bond? The number of unpaired electrons in the B2+ molecule is 2 (two) 1 (one) 3 (three) zero ; Question: Draw the molecular orbital diagram for B2+ (this is not B-B. it has an electron missing, so its B-B cation!) The number of unpaired electrons in the B2+ molecule is 2 (two) 1 (one) 3 (three) zero






















0 Response to "41 b2 2- molecular orbital diagram"
Post a Comment