41 hf molecular orbital diagram
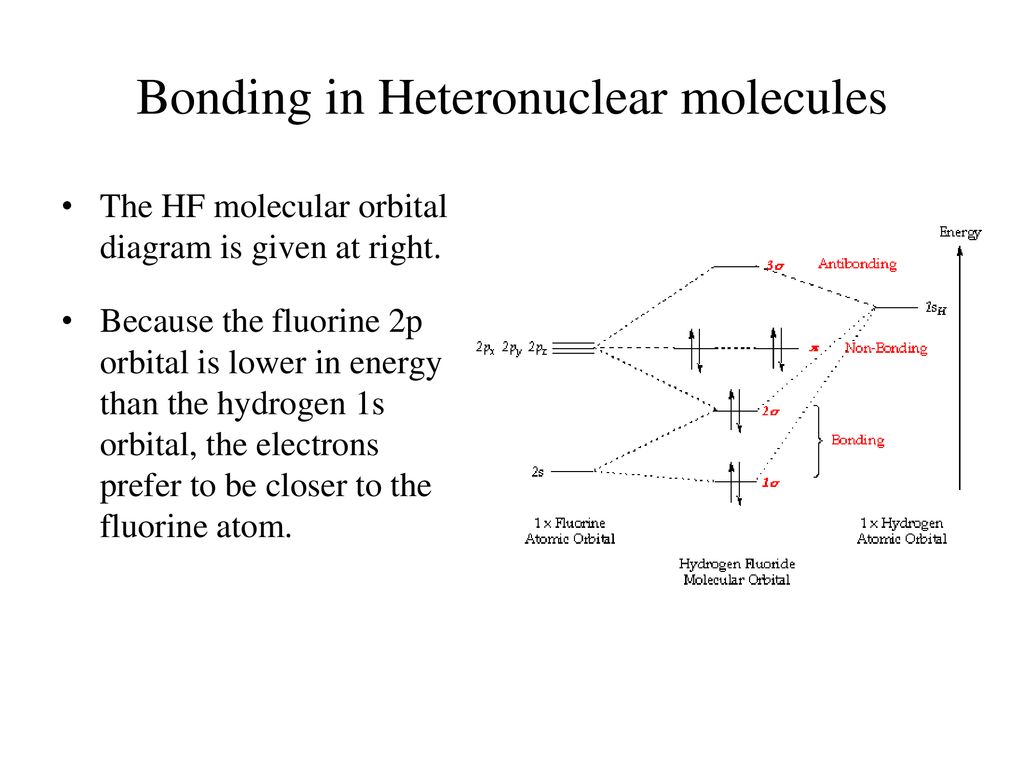
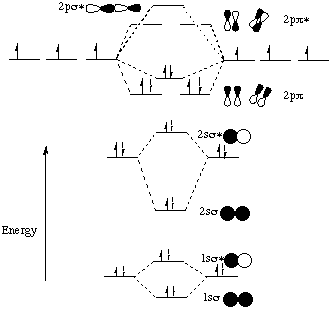
Which is the molecular orbital diagram for HF? The electronic of hydrogen and fluorine are 1s¹ and 1s²2s²2p⁵ respectively. In the formation of HF molecule ,only 2p electrons of fluorine atom would combine effectively with the solitary electron of hydrogen atom. As has been already explained ,only a pz orbital is able to combine with s orbital. In this screencast, Andrew Burrows walks you through how to construct the MO energy level diagram of HF. http://ukcatalogue.oup.com/product/9780199691852.do#...
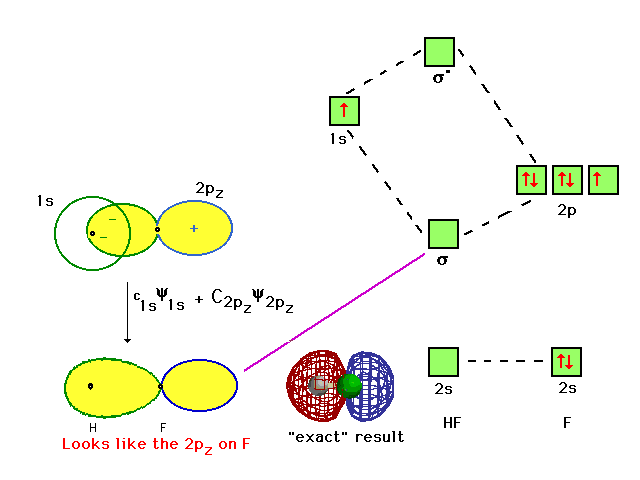
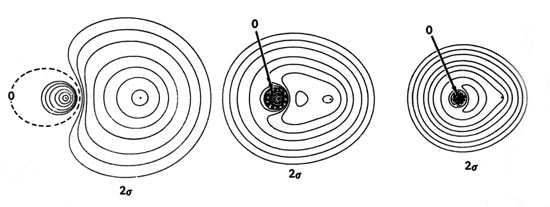
Molecular Orbital Diagram for the HF Molecule. Interaction occurs between the 1s orbital on hydrogen and the 2p orbital in fluorine causing the formation of a sigma-bonding and a sigma-antibonding molecular orbital, as shown below. Figure 1: Molecular orbitals of HF. (CC BY-SA-NC 2.0 UK: England & Wales License; Nick Greeves).
Hf molecular orbital diagram
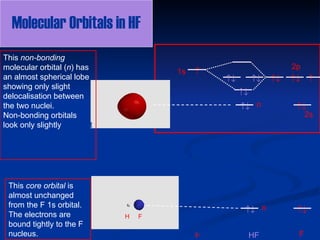
#MOT #BMO #ABMO #HF #CO #NO #CN #OHHello everyoneThis is shivam here To follow me on instagram search - Sshivam898To join telegram group click on the given l... Home / Structure and Bonding / Atomic Orbitals / Molecular orbitals in Hydrogen Fluoride. Molecular orbitals in Hydrogen Fluoride. CONTROLS . Click on the HF molecular orbitals in the energy level diagram to display the shapes of the orbitals. Explore bonding orbitals in other small molecules. Answer (1 of 3): The electronic of hydrogen and fluorine are 1s¹ and 1s²2s²2p⁵ respectively. In the formation of HF molecule ,only 2p electrons of fluorine atom would combine effectively with the solitary electron of hydrogen atom. As has been already explained ,only a pz orbital is able to combi...
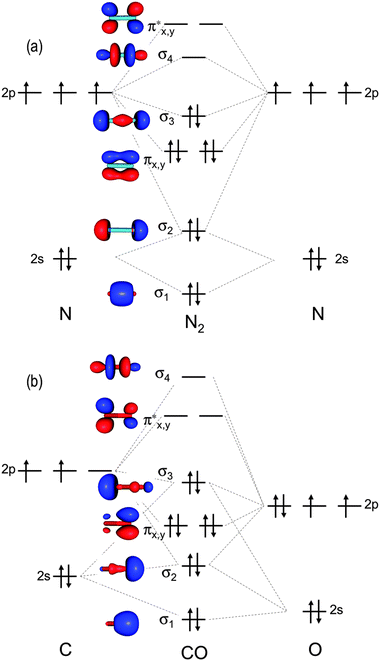
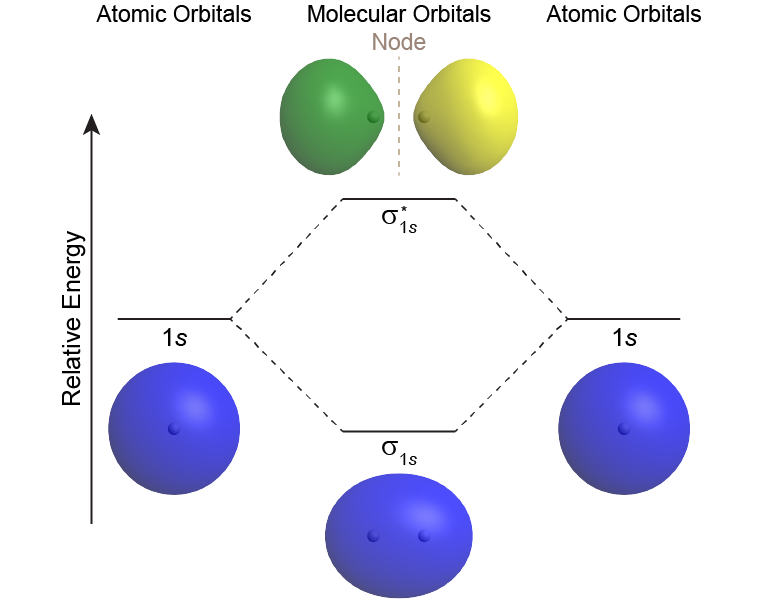
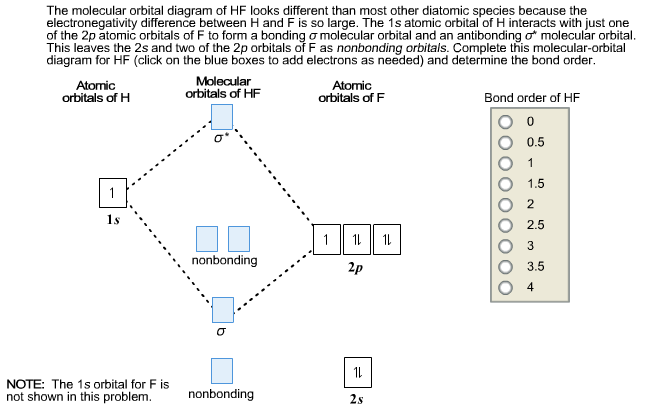
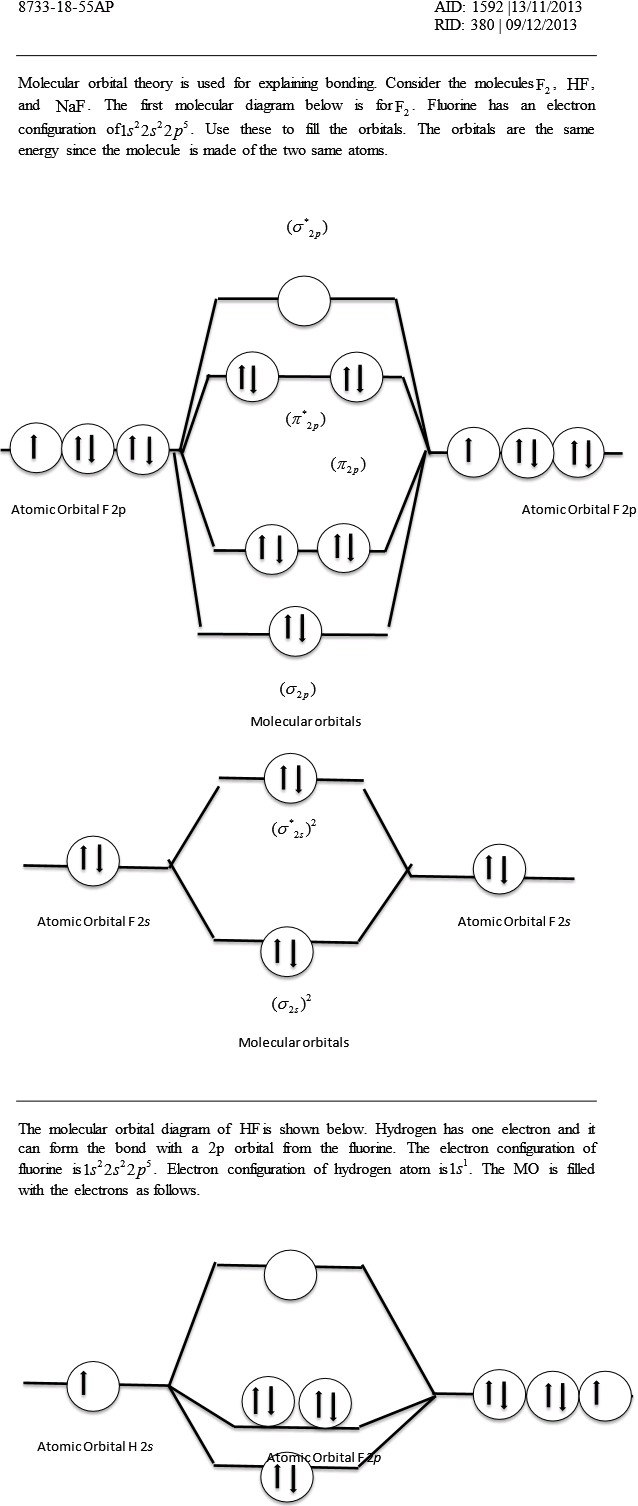
Hf molecular orbital diagram. Construct the molecular orbital diagram for HF. Use only the valence electrons for your diagram. The 2s orbital of F atom has an energy more than 26 eV lower than that of the H 1s, so there is very little interaction between them. The F 2p orbital (-18.65 eV) and the H 1s (-13.61 eV), on the other hand, have similar energies, allowing them to HF Molecular Orbital Diagram. Hydrogen fluoride is another example of a heteronuclear molecule. It is slightly different in that the π orbital is non-bonding, as well as the 2s σ. From the hydrogen, its valence 1s electron interacts with the 2p electrons of fluorine. This molecule is diamagnetic and has a bond order of one. Also, 1 π electrons are completely localised on the F atom because the 2 p x and 2 p y orbitals on F have a zero net overlap with the 1 s orbital on H. Electrons in MOs localized on a single atom are referred to as nonbonding electrons. Also, I would note that the 3 σ MO has less bonding character and the 4 σ ∗ MO has less anti-bonding ... Download scientific diagram | Molecular orbital diagrams for HBr and HF. from publication: Total energy partitioning within a one-electron formalism: A Hamilton population study of surface–CO ...
Answer (1 of 3): The electronic of hydrogen and fluorine are 1s¹ and 1s²2s²2p⁵ respectively. In the formation of HF molecule ,only 2p electrons of fluorine atom would combine effectively with the solitary electron of hydrogen atom. As has been already explained ,only a pz orbital is able to combi... Home / Structure and Bonding / Atomic Orbitals / Molecular orbitals in Hydrogen Fluoride. Molecular orbitals in Hydrogen Fluoride. CONTROLS . Click on the HF molecular orbitals in the energy level diagram to display the shapes of the orbitals. Explore bonding orbitals in other small molecules. #MOT #BMO #ABMO #HF #CO #NO #CN #OHHello everyoneThis is shivam here To follow me on instagram search - Sshivam898To join telegram group click on the given l...




















0 Response to "41 hf molecular orbital diagram"
Post a Comment