41 in this phase diagram for water indicate the direction that the solid-liquid and liquid-gas
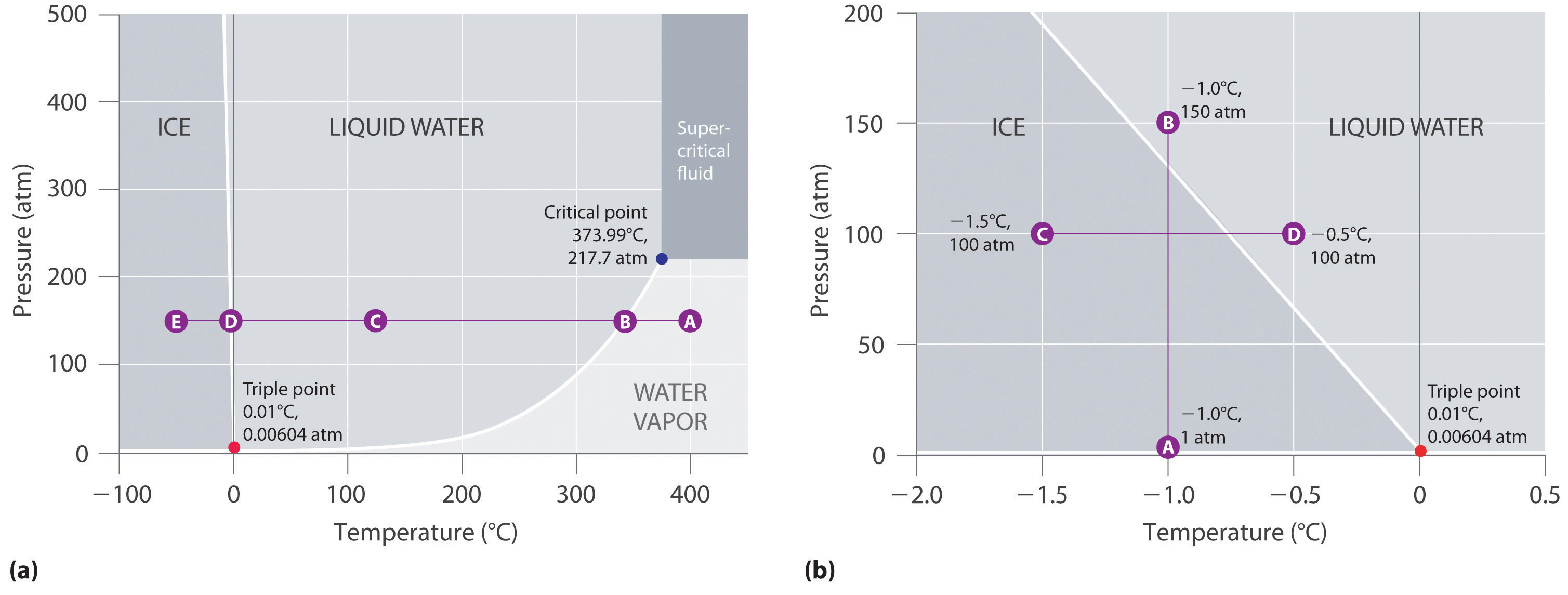
The water molecules are actually closer together in the liquid phase than they are in the solid phase. Refer again to water’s phase diagram ( Figure above ). Notice point E, labeled the critical point . What does that mean? At 373.99°C, particles of water in the gas phase are moving very, very rapidly. At any temperature higher than that ... Founded in 2002 by Nobel Laureate Carl Wieman, the PhET Interactive Simulations project at the University of Colorado Boulder creates free interactive math and science simulations. PhET sims are based on extensive education research and engage students through an intuitive, game-like environment where students learn through exploration and discovery.
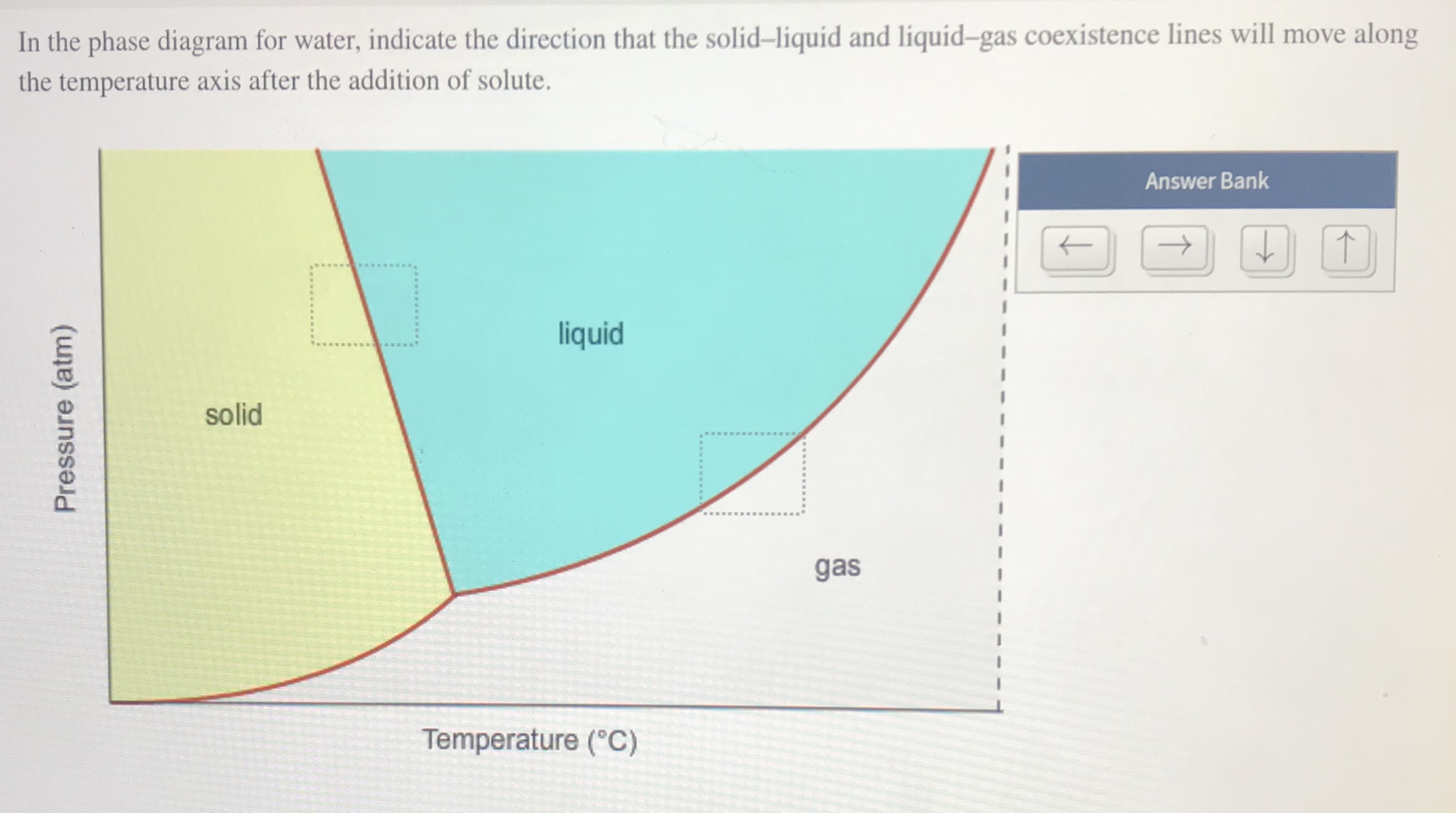
In this phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move after the addition of solute. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high.

In this phase diagram for water indicate the direction that the solid-liquid and liquid-gas
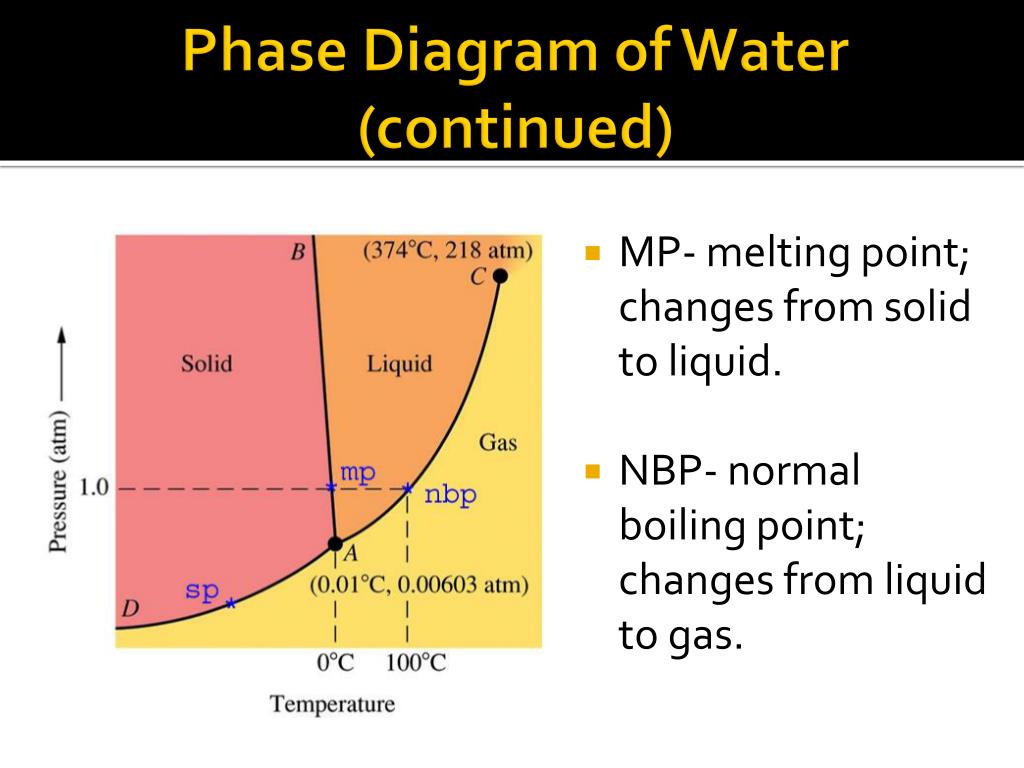
When you mix oil and water, as in the picture of the salad dressing below, the two materials will eventually separate because they do not mix well. They are immiscible. When they separate, the oil will always float on top. The two separate layers of water and oil are referred to as 'phases', the oil phase and the water phase. In this phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move after the addition of solute. Q. Label the diagram of pure solvent and a solution. A phase diagram combines plots of pressure versus temperature for the liquid-gas, solid-liquid, and solid-gas phase-transition equilibria of a substance. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure dependence of the phase-transition temperatures ...
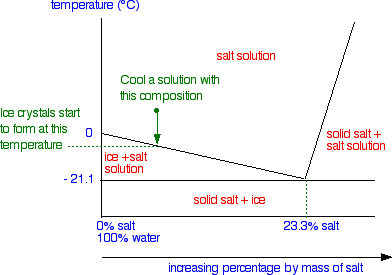
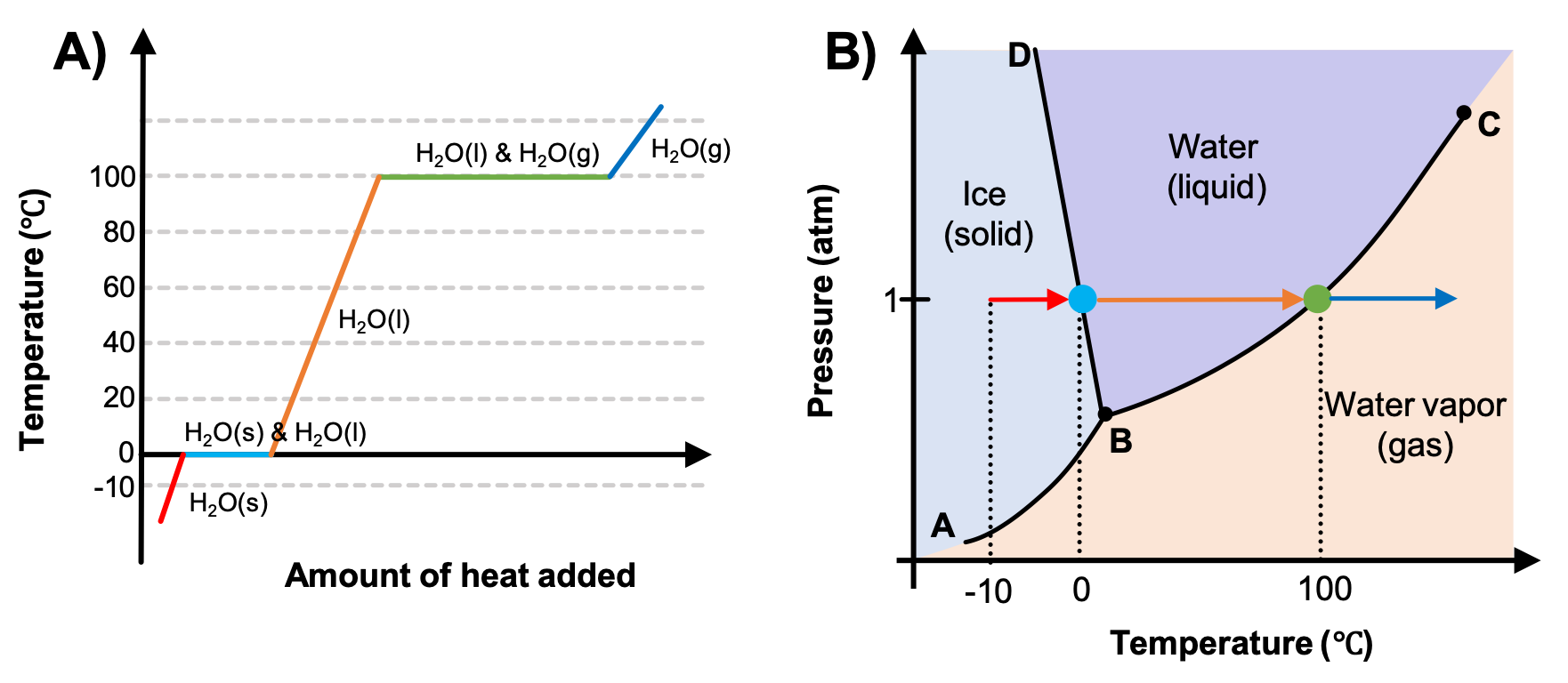
In this phase diagram for water indicate the direction that the solid-liquid and liquid-gas. Phase diagram of water Note: for H2O melting point decreases with increasing pressure, for CO2 melting point increases with increasing pressure. ... Melting point - temperature at which a substance turns from solid to liquid. solid liquid gas. The three phase changes can be brought about by changes in temperature or pressure: Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases. A 2.050×10−2 M solution of NaCl in water is at 20.0∘C. The sample was created by dissolving a sample of NaCl in water and then bringing the volume up to 1.000 L. It was determined that the volume of water needed to do this was 999.4 mL . The density of water at 20.0∘C is 0.9982 g/mL. Part A Calculate the molality of the salt solution. Figure 2. A heating curve and phase diagram for water. Solution. While anywhere along the line segment BD represents a phase change from solid to liquid, and points Y and Z are both on that line, the correct answer is D. At point Y, the phase change is occurring at the same pressure (1 atm) that was used to construct the heating curve.
In this phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move after the addition of solute. 15. Menthol is a crystalline substance with a peppermint taste and odor. If this is done with water, the partial pressure of water P w in the space above the liquid will initially be zero (step 1). Gradually, P w will rise as molecules escape from the liquid phase and enter the vapor phase. At the same time, some of the vapor molecules will condense back into the liquid phase (step 2). In the phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move along the temperature axis after the addition of solute. Calculate the osmotic pressure of a 0.186 M aqueous solution of sucrose, C12H22O11, at 37*C. Academia.edu is a platform for academics to share research papers.
Problem. : In this phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move after the addition of solute. FREE Expert Solution. After the addition of solute, the solid - liquid coexistence line will move towards lower temperatures. 95% (314 ratings) 1. Recall the different states of matter (solid, liquid, gas). How do the water molecules differ in the liquid and gas states? Draw and explain. 2. Plot your temperature and time data on the graph below. 3. Label the phase changes on your heating curve above. 4. What happens to the molecules as they begin to boil? 5. Aug 01, 2014 · The applications of PCMs with a solid–gas or liquid–gas phase transition are limited in TES systems because of the large volume changes associated with the transition – even if they possess a high phase transition latent heat .Significantly smaller volume changes occur, usually ca. 10% or less, with solid–solid and solid–liquid transformations. In chemistry, thermodynamics, and many other related fields, phase transitions (or phase changes) are the physical processes of transition between a state of a medium, identified by some parameters, and another one, with different values of the parameters. Commonly the term is used to refer to changes among the basic states of matter: solid, liquid, and gas, as well as plasma …
Chemistry questions and answers In the phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move along the temperature axis after the addition of solute. Answer Bank 1. → liquid Pressure (atm) solid gas Temperature (°C)
A phase diagram combines plots of pressure versus temperature for the liquid-gas, solid-liquid, and solid-gas phase-transition equilibria of a substance. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure dependence of the phase-transition temperatures ...
In this phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move after the addition of solute. Q. Label the diagram of pure solvent and a solution.
When you mix oil and water, as in the picture of the salad dressing below, the two materials will eventually separate because they do not mix well. They are immiscible. When they separate, the oil will always float on top. The two separate layers of water and oil are referred to as 'phases', the oil phase and the water phase.

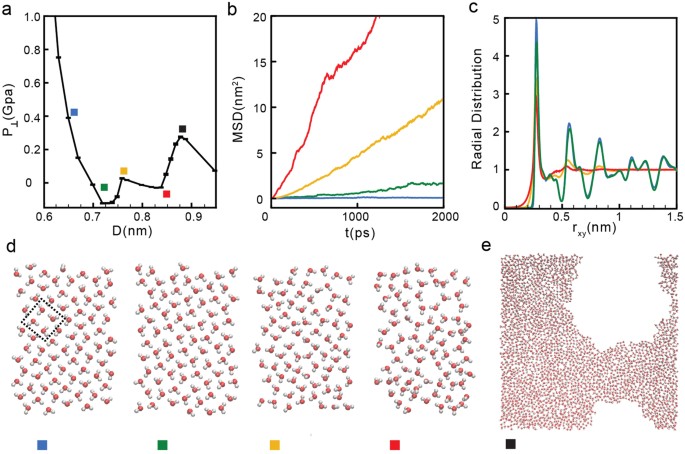








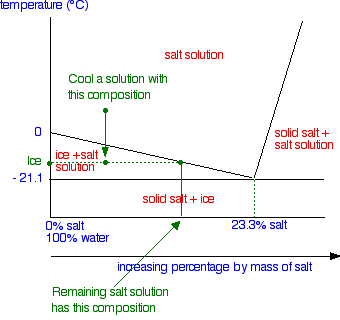

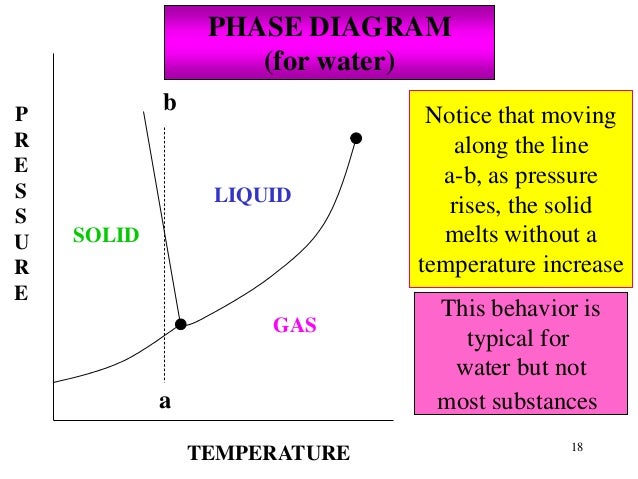


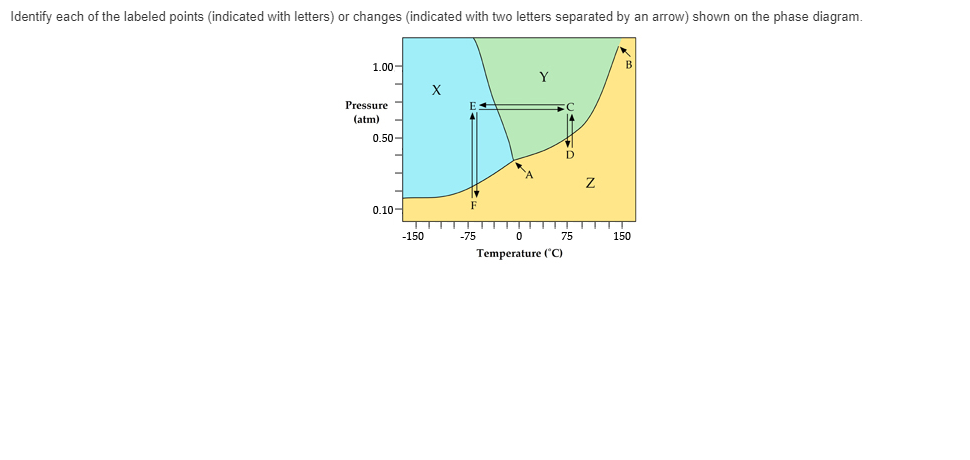











0 Response to "41 in this phase diagram for water indicate the direction that the solid-liquid and liquid-gas"
Post a Comment