42 orbital diagram for phosphorus
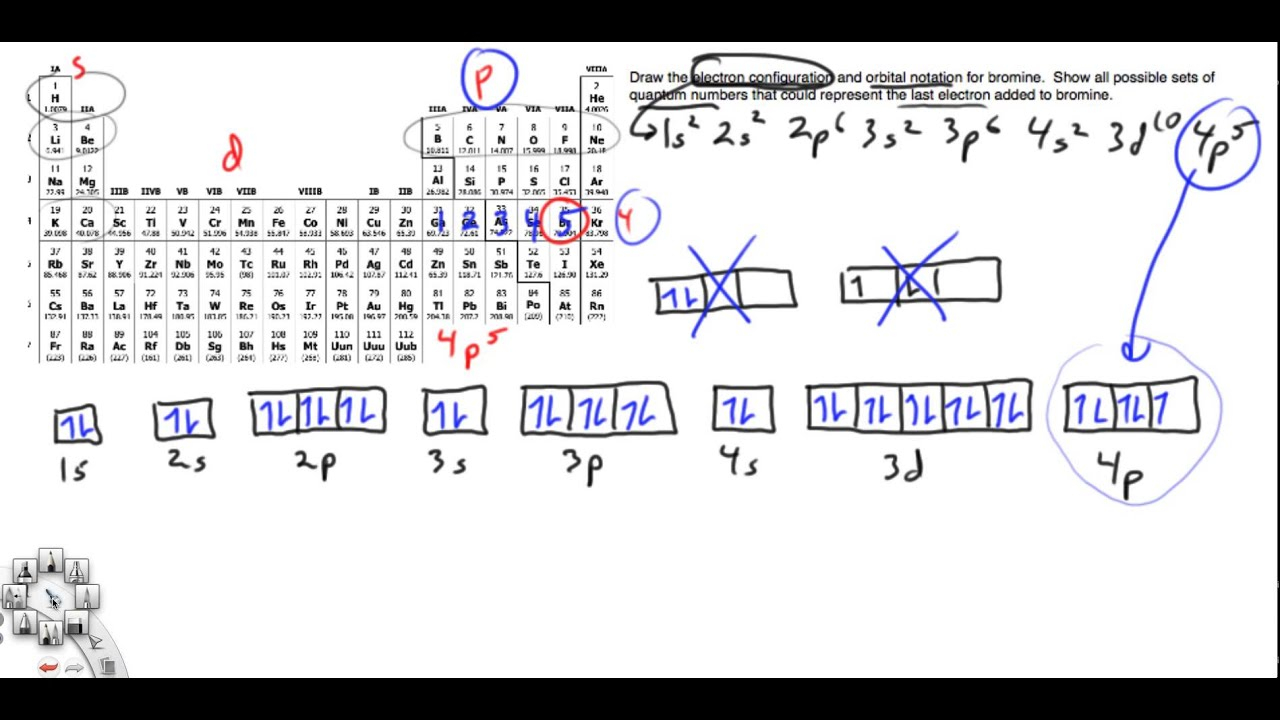
Aug 14, 2020 · What is the electron configuration and orbital diagram for a phosphorus atom? What are the four quantum numbers for the last electron added? Solution. The atomic number of phosphorus is 15. Thus, a phosphorus atom contains 15 electrons. The order of filling of the energy levels is 1s, 2s, 2p, 3s, 3p, 4s, . . . Electron configuration was first conceived under the Bohr model of the atom, and it is still common to speak of shells and subshells despite the advances in understanding of the quantum-mechanical nature of electrons.. An electron shell is the set of allowed states that share the same principal quantum number, n (the number before the letter in the orbital label), that electrons …
A step-by-step description of how to write the electron configuration for Phosphorus (P). In order to write the P electron configuration we first need to kn...
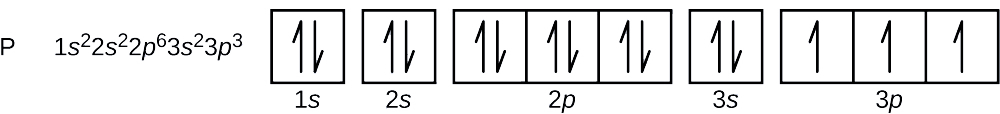
Orbital diagram for phosphorus
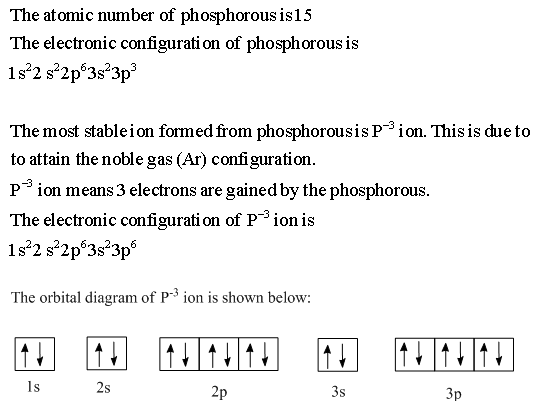
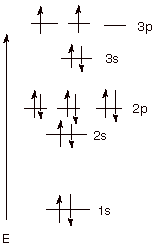
Orbital diagram. Phosphorus electron configuration ← Electronic configurations of elements . P (Phosphorus) is an element with position ... 1s 2 2s 2 2p 6 3s 2 3p 3 Reduced electronic configuration P: [Ne] 3s 2 3p 3. Below is the electronic diagram of the Phosphorus atom Distribution of electrons over energy levels in the P atom 1-st level (K ... orbital diagram (orbital box diagram) : Pairs of electrons occupy the 1s, 2s, 2p x, 2p y, 2p z, 3s, 3p x, 3p y, 3p z and 4s orbital, and only 1 electron occupies each of the 3d orbitals and these electrons have parallel spin (arrows pointing in the same direction) in accordance with Hund's Rule. Apr 10, 2018 · Uml Class Diagram Notation Cheat Sheet; Ps4 Airflow Diagram; 99 Dodge Dakota Sport 3.9 Pcm Wiring Diagram; Cm7000 Wiring Diagram; Eaton C440 Overload Relay Wiring Diagram; Hive Active Heating Wiring Diagram; Gri 6644 Wiring Diagram; Orbital Box Diagram Phosphorus; Ddec 4 Wiring Diagram; Recent Comments. Bbhank on Western plow ultra mount wiring ...
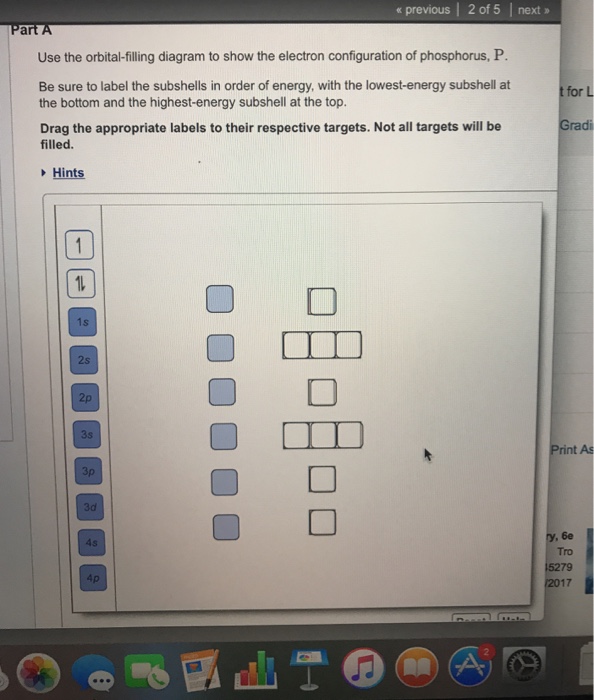
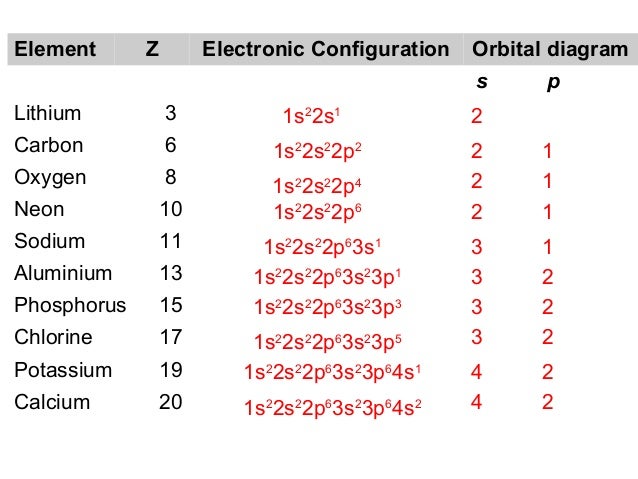
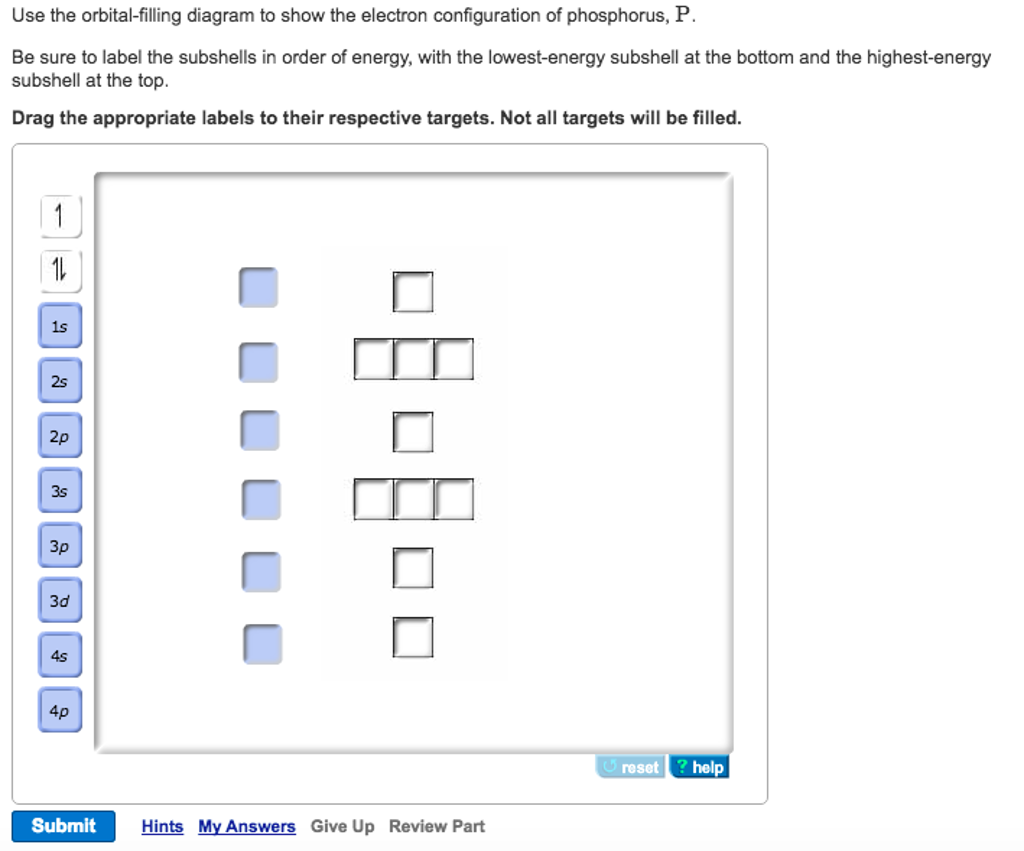
Orbital diagram for phosphorus. The aufbau diagram shows the. The atomic number of phosphorus is Write the electron configuration of a phosphorus atom. 1s22s22p63s23p3. You can obtain correct electron configurations for the elements up to. In writing the electron configuration for Phosphorus the first two electrons will go in the 1s orbital. 30 May 2020 — The electron configuration for phosphorus is 1s2 2s2 2p 6 3s 2 3p 3 and the orbital diagram is drawn below. orbial diag phosphorus.png. Back to ...Introduction · Rules for Assigning Electron... · Pauli Exclusion Principle What is the electron configuration and orbital diagram for a phosphorus atom? What are the four quantum numbers for the last electron added? Solution The atomic number of phosphorus is 15. Thus, a phosphorus atom contains 15 electrons. The order of filling of the energy levels is 1s, 2s, 2p, 3s, 3p, 4s, . . . Orbital Diagram Of Phosphorus. what is the orbital diagram for phosphorus the orbital diagram for phosphorus consists of five electrons in the third shell eight in the second and two in the first shell closest to the nucleus electron configuration for phosphorus p the next six electrons will go in the 2p orbital the p orbital can hold up to six electrons we ll put six in the 2p orbital and ...
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ... 21 Jan 2021 — Phosphorous is a chemical element that has an atomic number of 15. Its electron configuration with regards to electrons present in each shell is ... Phosphorus (P) has an atomic mass of 15. Find out about its chemical and ... Electron Configuration, [Ne] 3s2 3p3. 1s2 2s2 2p6 3s2 3p3. Orbital Diagram. Jan 01, 2022 · Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13: Orbital diagram of Aluminum (Al) 14: Orbital diagram of Silicon (Si) 15: Orbital diagram of Phosphorus (P) 16: Orbital diagram of Sulfur (S) 17: Orbital diagram of Chlorine (Cl) 18: Orbital diagram of ...
Build the orbital diagram for the ion most likely formed by phosphorus? 1s22s22p63s23p3 is for Phosphorus and the most likely ion is to be a 3- because it wants to have a full outer shell ... By knowing any two values of the Voltage, Current or Resistance quantities we can use Ohms Law to find the third missing value.Ohms Law is used extensively in electronics formulas and calculations so it is “very important to understand and accurately remember these formulas”.. To find the Voltage, ( V ) [ V = I x R ] V (volts) = I (amps) x R (Ω) 03-12-2021 · Vanadium-phosphorus incorporation induced interfacial modification on ... corresponding to Co 2p 3/2 (780–782 eV) and Co 2p 1/2 (796–799 eV), attributing to the spin-orbit splitting of the p orbital along with two shakeup ... Co-P surfaces for the OER process. (C) The Free Energy diagram for each elemental step of OER ... The newly produced hybrid orbitals in PF3 molecule have 25% behavior of the s orbital and 75% behavior of the p orbital. The bond angle of PF3 “Phosphorus trifluoride has an F−P−F bond angle of approximately 96°“.
Therefore the Phosphorus electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 3. Furthermore, what is an atomic orbital diagram? Orbital diagrams are pictorial descriptions of the electrons in an atom. Three rules are useful in forming orbital diagrams. According to the Auf Bau Principle, each electron occupies the lowest energy orbital. The ...
The atomic number of boron is 5 and its symbol is ‘B’. The standard atomic mass of boron is 10.806. The period of boron is 2 and it is a p-block element. This article gives an idea about the electron configuration of boron(B) and orbital diagram, period and groups, valency and valence electrons of boron, application of different principles.
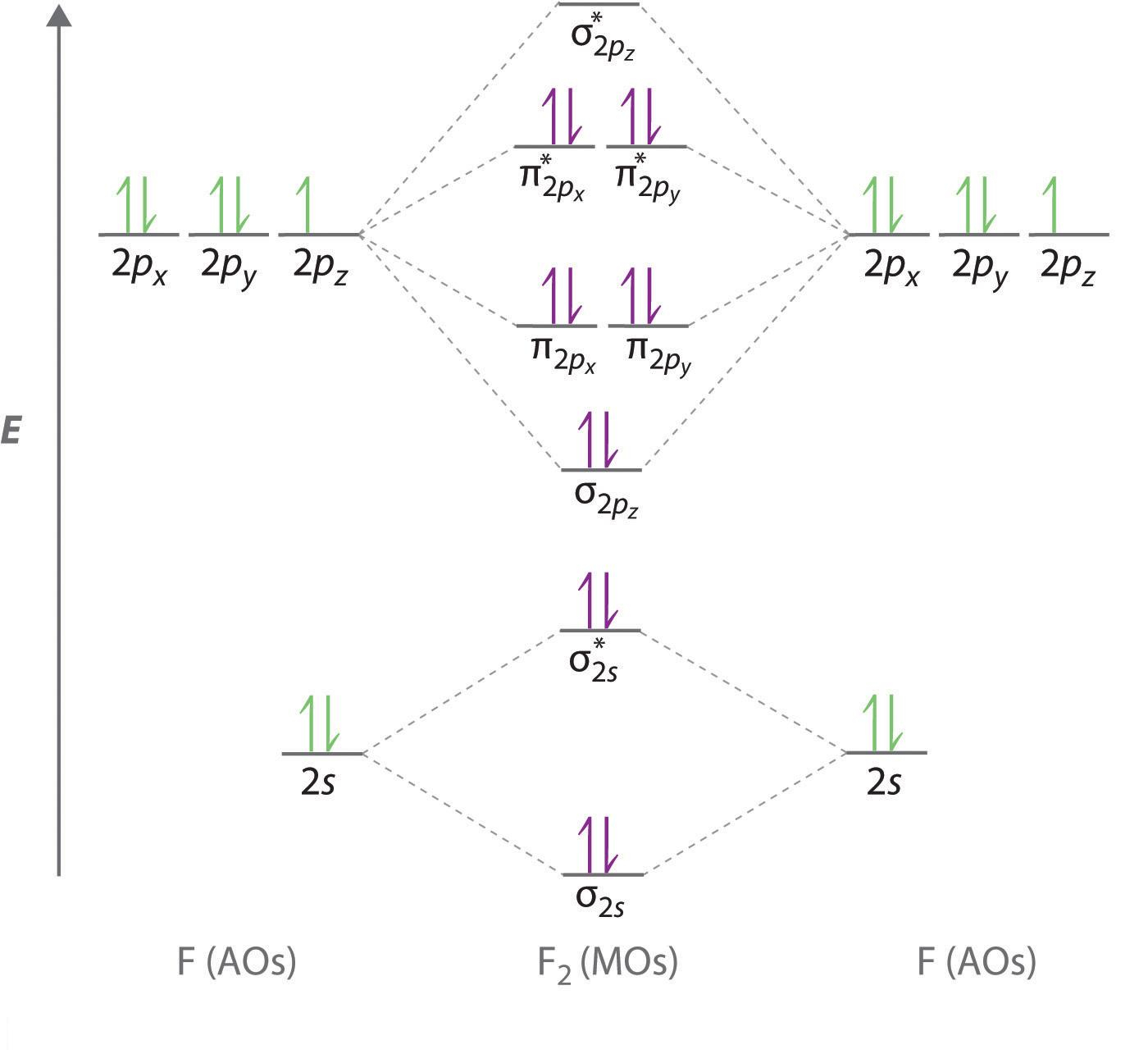
A molecular orbital diagram or MO diagram for short is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the Linear combination of atomic orbitals molecular orbital method (LCAO method) in particular.schematron.org: Phosphorus: Orbital and Bonding InfoWhat is the orbital ...
The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. The energy increases as we move up to the 2s and then 2p, 3s, and 3p orbitals, showing that the increasing n value has more influence on energy than the increasing l value for small atoms.
Phosphorus orbital diagram. Paired Unpaired. Thus a phosphorus atom contains 15 electrons. What are the four quantum numbers for the last electron added. Since 1s can only hold two electrons the next 2 electrons for Phosphorous go in the 2s orbital. Visualizing the diagram we come up with a Phosphorus in the center housed by 5 Chlorine atoms.
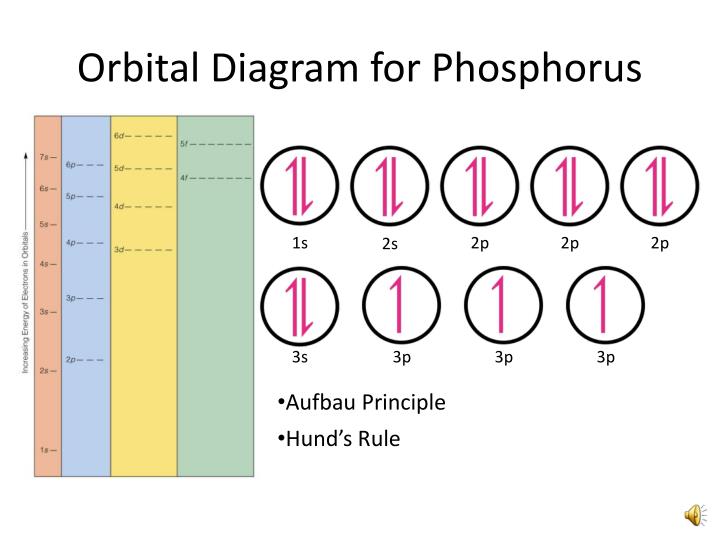
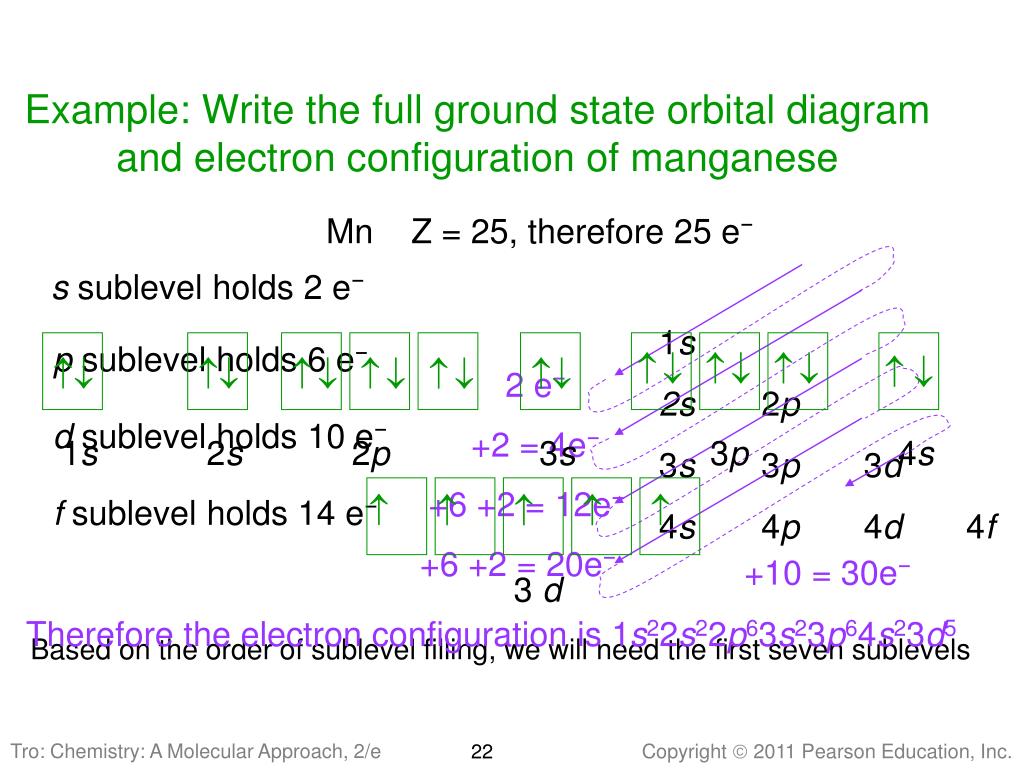
To create an orbital diagram of an atom, you first need to know Hund's principle and Pauli's exclusion principle. Hund's principle is that electrons in different orbitals with the same energy would be positioned in such a way that they could be in the unpaired state of maximum number and the spin of the unpaired electrons will be one-way.
Locate the nearest noble gas preceding phosphorus in the periodic table. Then subtract its number of electrons from those in phosphorus to obtain the number of valence electrons in phosphorus. Referring to Figure , draw an orbital diagram to represent those valence orbitals. In writing the electron configuration for Phosphorus the first two ...
Molecular orbital theory (MO theory) provides an explanation of chemical bonding that accounts for the paramagnetism of the oxygen molecule. It also explains the bonding in a number of other molecules, such as violations of the octet rule and more molecules with more complicated bonding (beyond the scope of this text) that are difficult to describe with Lewis structures.
In writing the electron configuration for Phosphorus the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Phosphorous go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the ...
What is the orbital diagram for phosphorus? In writing the electron configuration for Phosphorus the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Phosphorous go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons.
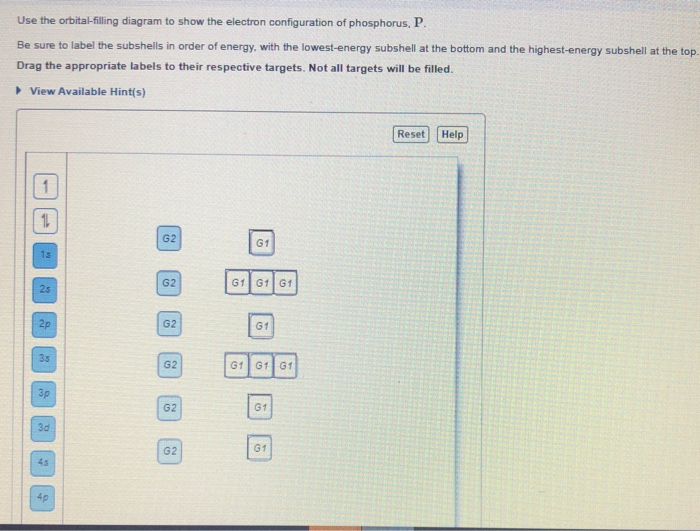
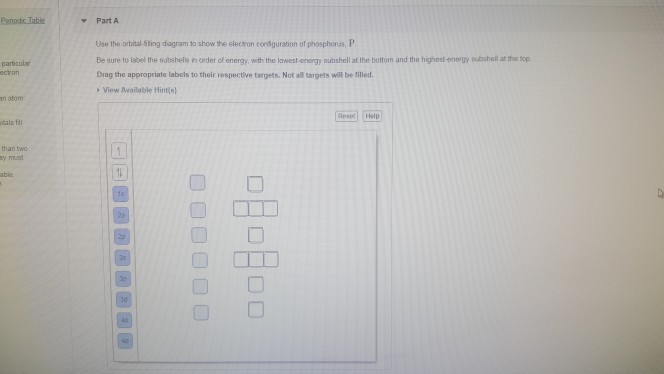
Chemistry. Chemistry questions and answers. Part A Choose the orbital diagram for phosphorus. 11 11 Is 11 2s 101L 1L 2p 11 35 3p 11 11 11 111111 2p Is 2s 3s Зр 10 IL Is LILL 2p 11 3s 25 3p 11 Is 11 25 111111 2p 11 3s 3p Part B Determine the number of unpaired electrons. Express your answer as an integer.
5 Mar 2015 · 1 answerBefore writing any quantum numbers for phosphorus' five valence electrons, let's write the atom's electron configuration, since this will ...
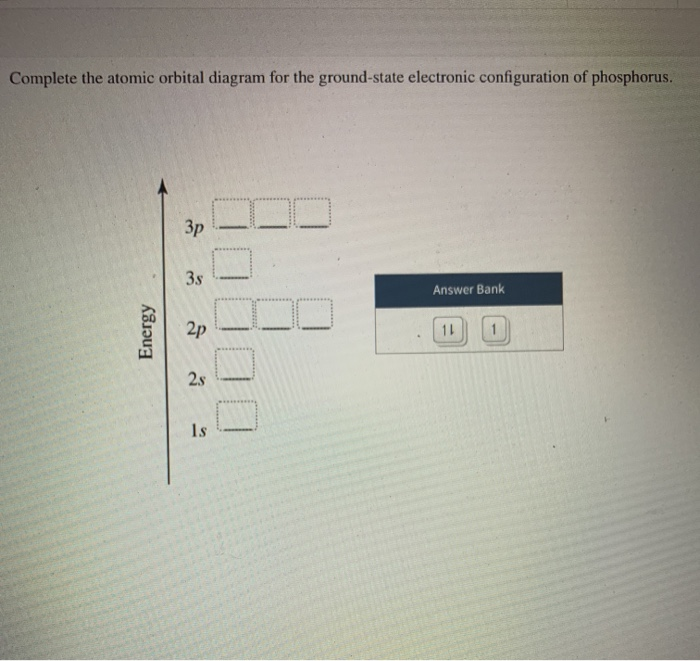
science. chemistry. chemistry questions and answers. Fill In The Orbital Energy Diagram For Phosphorus. 3p 2p.
Molecular orbital diagram ne2 28.12.2018 28.12.2018 7 comments on molecular orbital diagram ne2 even rather simple molecular orbital (mo) theory can be used to predict from the bottom of the diagram because this is how mo diagrams are constructed, from n2, o2, f2, ne2 the complexity of the molecular orbitals develop in two ways.
28-12-2021 · PCl3 Molecular Orbital (MO) Diagram. In a molecular orbital diagram of PCl3, we can see 3 bonding orbitals, which will be occupied. And 3 anti-bonding orbitals which will be empty. We can see from the hybridization that 3 Sp3 hybrid orbitals of phosphorus will be occupied by 3 Cl atoms.
To write the orbital diagram for the Phosphorus atom (P) first we need to write the electron configuration for just P. To do that we need to find the number...
Answer to: Draw and explain the orbital diagram for phosphorus. By signing up, you'll get thousands of step-by-step solutions to your homework...
(Recall from Section 5.3B that two electrons in an orbital spin in opposite directions on their axes.) Therefore, if an orbital contains two electrons, its box will contain two arrows, one pointing up and the other down. Using a box diagram, we show the electron configuration of nitrogen as: Notice that the 2p electrons are shown as
The electron configuration of fluorine(F) and the orbital diagram is the main topic of this article. This article gives an idea about the fluorine orbital diagram, period and groups, valency and valence electrons of fluorine, bond formation, compound formation, application of different principles.
Description. The sun, which drives the water cycle, heats water in the ocean and seas. Water evaporates as water vapor into the air.Some ice and snow sublimates directly into water vapor. Evapotranspiration is water transpired from plants and evaporated from the soil. The water molecule H 2 O has smaller molecular mass than the major components of the atmosphere, …
The whole point of that was to see how the oxygen orbital energies split up (and which were two-fold or three-fold degenerate). It was also to figure out which orbitals on phosphorus interact with which orbitals on the oxygen atoms. The resultant MO diagram was: Takeaways: This is only qualitative, so take it with a grain of salt.
Apr 10, 2018 · Uml Class Diagram Notation Cheat Sheet; Ps4 Airflow Diagram; 99 Dodge Dakota Sport 3.9 Pcm Wiring Diagram; Cm7000 Wiring Diagram; Eaton C440 Overload Relay Wiring Diagram; Hive Active Heating Wiring Diagram; Gri 6644 Wiring Diagram; Orbital Box Diagram Phosphorus; Ddec 4 Wiring Diagram; Recent Comments. Bbhank on Western plow ultra mount wiring ...
orbital diagram (orbital box diagram) : Pairs of electrons occupy the 1s, 2s, 2p x, 2p y, 2p z, 3s, 3p x, 3p y, 3p z and 4s orbital, and only 1 electron occupies each of the 3d orbitals and these electrons have parallel spin (arrows pointing in the same direction) in accordance with Hund's Rule.
Orbital diagram. Phosphorus electron configuration ← Electronic configurations of elements . P (Phosphorus) is an element with position ... 1s 2 2s 2 2p 6 3s 2 3p 3 Reduced electronic configuration P: [Ne] 3s 2 3p 3. Below is the electronic diagram of the Phosphorus atom Distribution of electrons over energy levels in the P atom 1-st level (K ...


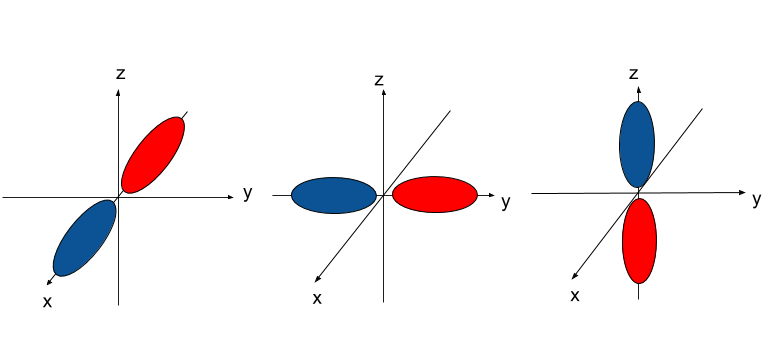


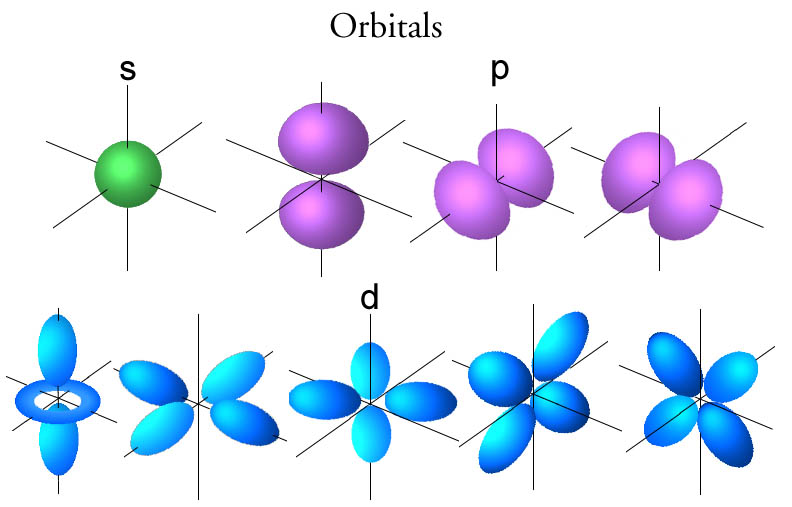
/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)









0 Response to "42 orbital diagram for phosphorus"
Post a Comment