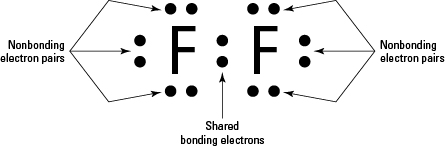
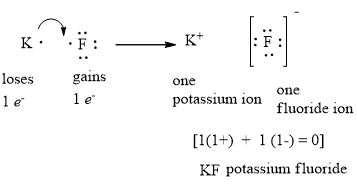
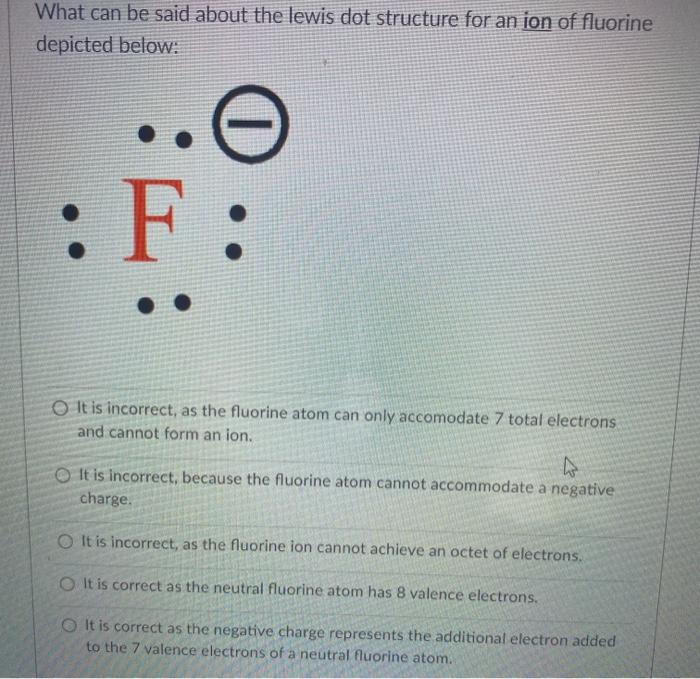
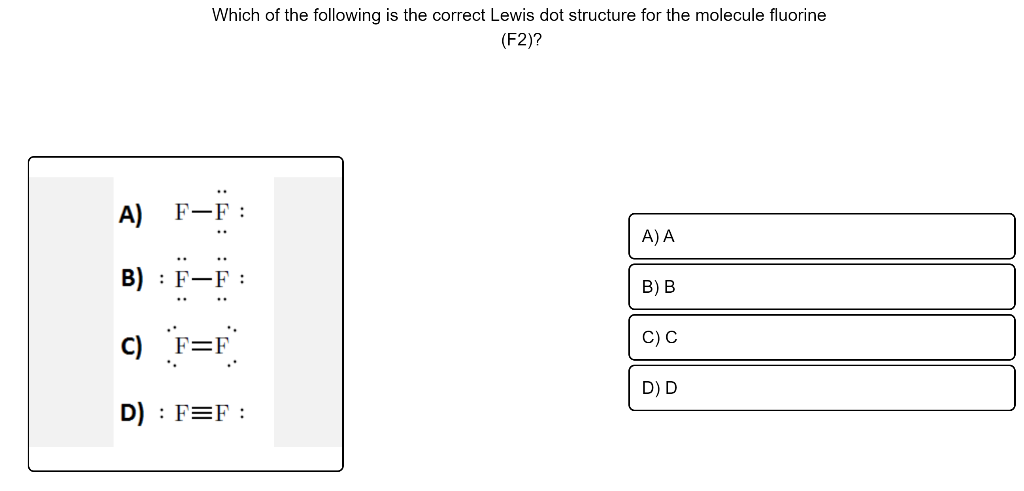
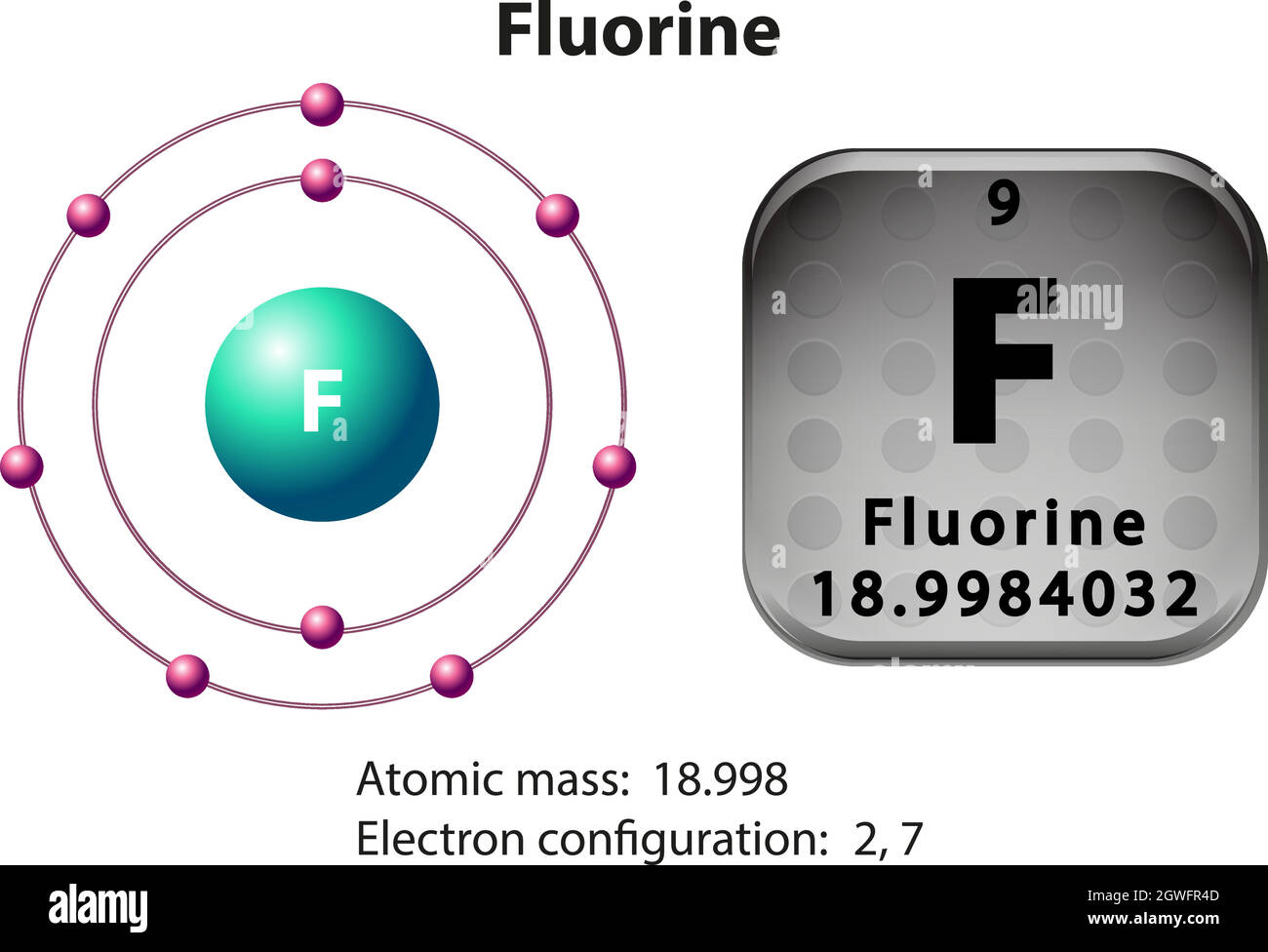
37 fluorine electron dot diagram
Beryllium–fluorine Help students fill in the correct number of electrons in the energy levels for the rest of the atoms in period 2. Period 3 Sodium Explain that sodium has 11 protons and 11 electrons. There are 2 electrons on the first energy level, 8 electrons on the second level, and 1 electron on the third energy level. molecule and are represented by Lewis dot structures. • Draw Lewis dot diagrams to represent valence electrons in elements and draw Lewis dot structures to show covalent bonding. • Use valence shell electron pair repulsion (VSEPR) model to draw and name molecular shapes (bent, linear, trigonal planar, tetrahedral, and trigonal pyramidal).
MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H–F nb σ σ* Energy H –13.6 eV 1s F –18.6 eV –40.2 eV 2s 2p So H–F has one σ bond and three lone electron pairs on fluorine

Fluorine electron dot diagram
4. Write the electron configuration _____ 5. Draw the orbital diagram: 6. Number of valence electrons _____ 7. Draw the Lewis dot diagram: S Directions For 8-14: Given the element Magnesium (Atomic Number 12, Mass Number 24), please provide all the following information for this element. 8. So, being more electronegativity of fluorine help it to attract more bonded electron than nitrogen. Therefore fluorine gains a partial negative charge and nitrogen gains a partial positive charge. Positive and negative charges cause non-uniform charge distribution around the NF3 structure. Hence it also causes NF3 to become polar in nature. Lewis Brisbois Bisgaard & Smith LLP (OR) 888 SW Fifth Avenue Suite 900 Portland, OR 97204 (971) 712-2802 . Curtis R. Webb Curtis R. Webb P. O. Box 429 Monmouth, OR 97361 (503) 623-7002. PR: PUERTO RICO. Roberto E. Ruiz-Comas RC Legal & Litigation Services PSC Suite 801 Resolucion St. No. 33
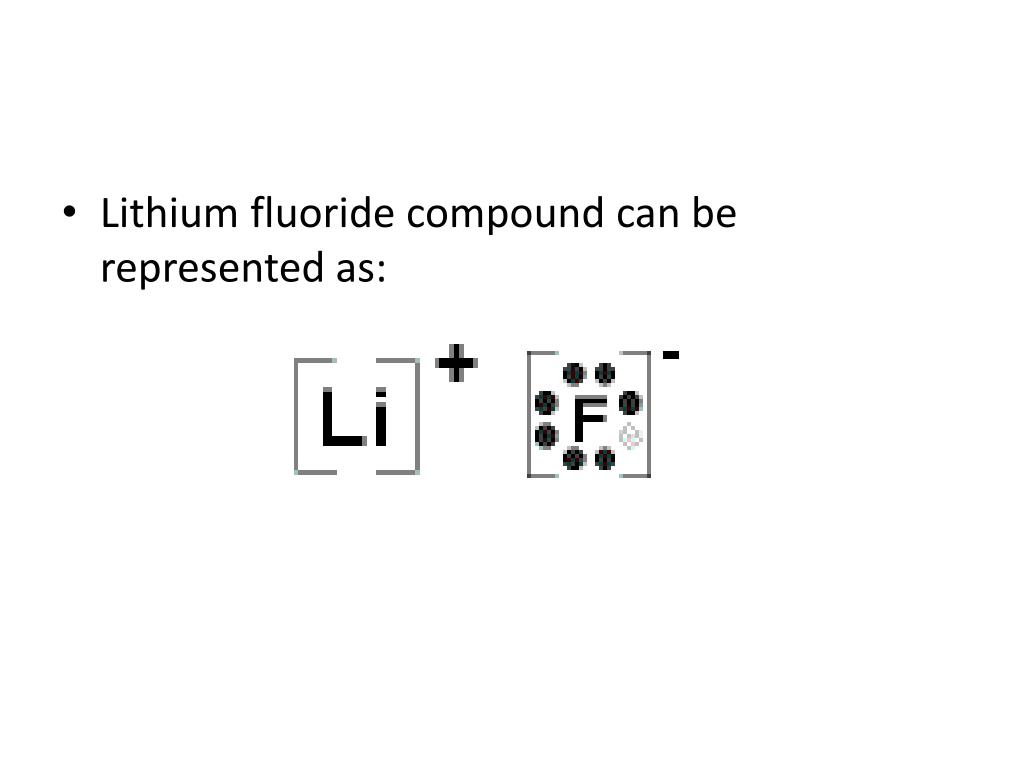
Fluorine electron dot diagram. May 06, 2018 · Dispersion Forces CO_2 has dispersion forces or van der waals forces as its only intermolecular force. Since CO_2 is made of one carbon and 2 oxygen and both carbon and oxygen are non-metals, it also have covalent bonds. For extra information, there are 3 types of intermolecular forces. Dispersion Forces Dipole-dipole Hydrogen bonds Dispersion forces are weaker than dipole-dipole and dipole ... The octet rule is a chemical rule of thumb that reflects the theory that main-group elements tend to bond in such a way that each atom has eight electrons in its valence shell, giving it the same electronic configuration as a noble gas. Lewis Brisbois Bisgaard & Smith LLP (OR) 888 SW Fifth Avenue Suite 900 Portland, OR 97204 (971) 712-2802 . Curtis R. Webb Curtis R. Webb P. O. Box 429 Monmouth, OR 97361 (503) 623-7002. PR: PUERTO RICO. Roberto E. Ruiz-Comas RC Legal & Litigation Services PSC Suite 801 Resolucion St. No. 33 So, being more electronegativity of fluorine help it to attract more bonded electron than nitrogen. Therefore fluorine gains a partial negative charge and nitrogen gains a partial positive charge. Positive and negative charges cause non-uniform charge distribution around the NF3 structure. Hence it also causes NF3 to become polar in nature.
4. Write the electron configuration _____ 5. Draw the orbital diagram: 6. Number of valence electrons _____ 7. Draw the Lewis dot diagram: S Directions For 8-14: Given the element Magnesium (Atomic Number 12, Mass Number 24), please provide all the following information for this element. 8.





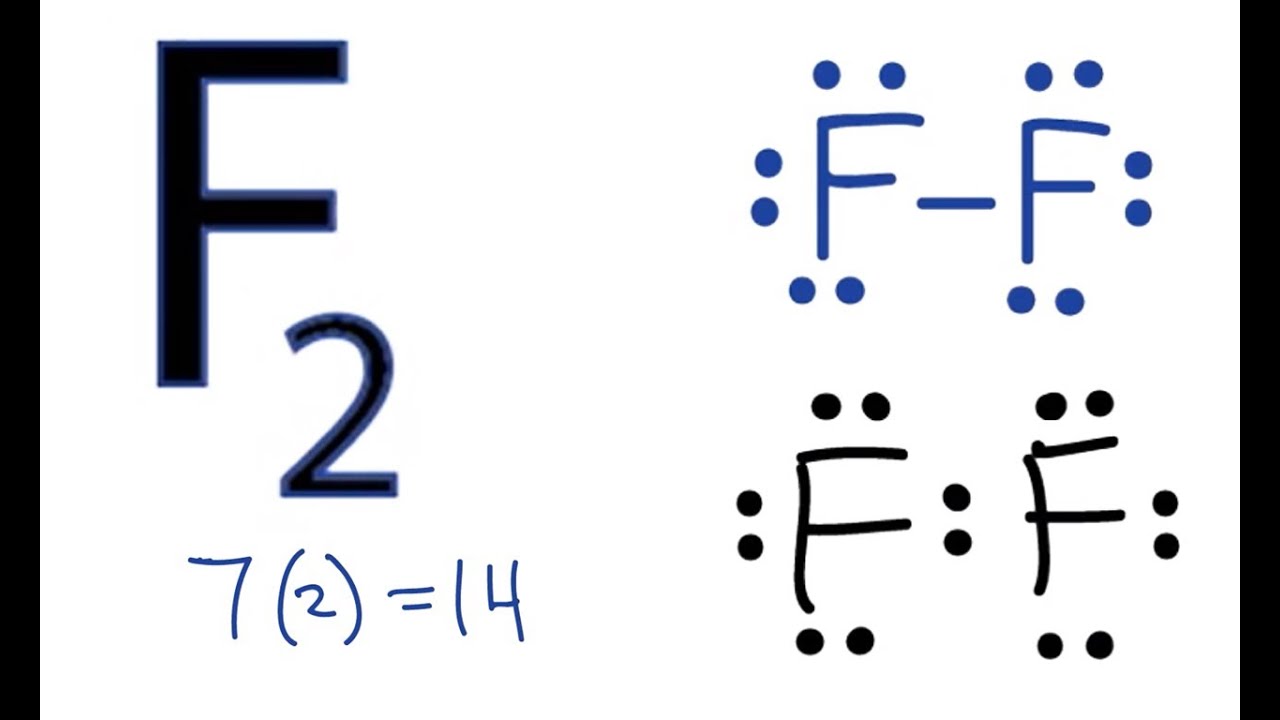









![Expert Answer] draw the electron dot structure of F2 - Brainly.in](https://hi-static.z-dn.net/files/df8/67df4fdbd3d368c6287c82f3548774aa.png)
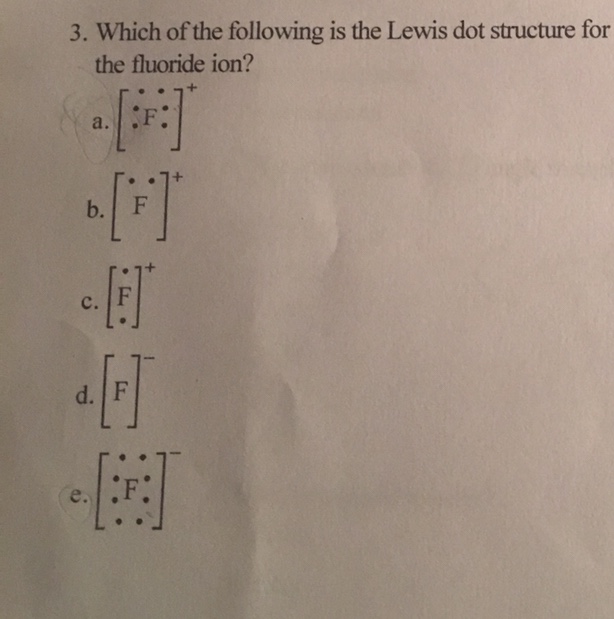








0 Response to "37 fluorine electron dot diagram"
Post a Comment