37 Use The Drawing Of The Mo Energy Diagram To Predict The Bond Order Of Li2+.
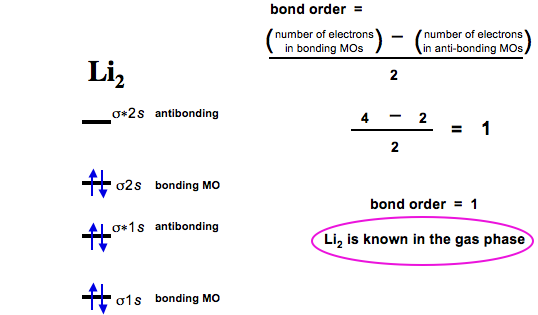
Why #"Li"_2^+# is more stable than #"Li"_2# - Socratic.org Li2 is more stable than Li+ 2, because the bond is (hypothetically) stronger (probably gas-phase). Here we consider the molecular orbital diagram (MO) of Li2: The bond order can be calculated in a simple manner. Just take electrons that are in each MO, and. for each electron in a bonding MO, it adds 0.5 to the bond order, because more bonding ... What is the bond order of Li2−? Is Li2− ... - Clutch Prep We're being asked to determine the bond order of Li2-. For this, we need to do the following steps: Step 1: Calculate the total number of valence electrons present. Step 2: Draw the molecular orbital diagram. Step 3: Calculate the bond order of the molecule/ion. Recall that the formula for bond order is: Bond Order = 1 2[# of e - in bonding ...
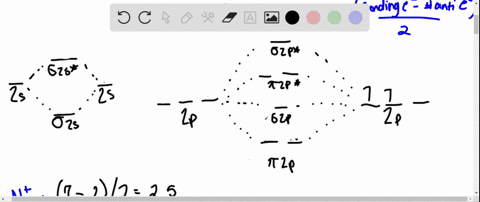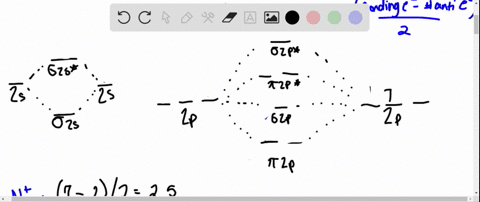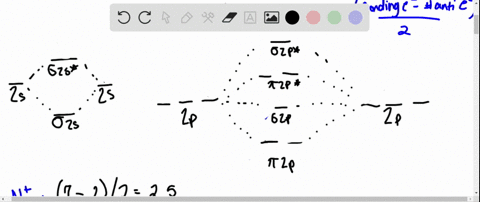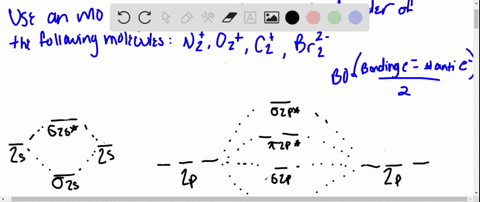
Solved Complete the MO energy diagram and predict the bond ... Complete the MO energy diagram and predict the bond order of Li_2^+ and Li_2^-. You may want to reference (pages 409 - 419) Section 10.8 while completing this problem. Complete the atomic orbital (AO) and molecular orbital (MO) energy diagram for Li_2^+ Drag the appropriate labels to their respective targets.

Use the drawing of the mo energy diagram to predict the bond order of li2+.
4.10: Second-Row Diatomic Molecules - Chemistry LibreTexts Figure 4.10.1: Molecular Orbital Energy-Level Diagrams for Homonuclear Diatomic Molecules. (a) For F 2, with 14 valence electrons (7 from each F atom), all of the energy levels except the highest, σ 2 p z ⋆ are filled. This diagram shows 8 electrons in bonding orbitals and 6 in antibonding orbitals, resulting in a bond order of 1. SOLVED:Draw an MO energy diagram and predict the bond ... Let's first draw the molecular orbital energy diagram where we have sigma to us at the bottom, then sigma to a star P two P, sigma two P. P two P. Star and sigma two P star. This is what we will see for beryllium, where these two are switched in comparison to the elements that are farther to the right on the periodic table. PDF MO Diagrams for Diatomic Molecules - UCI Department of ... MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
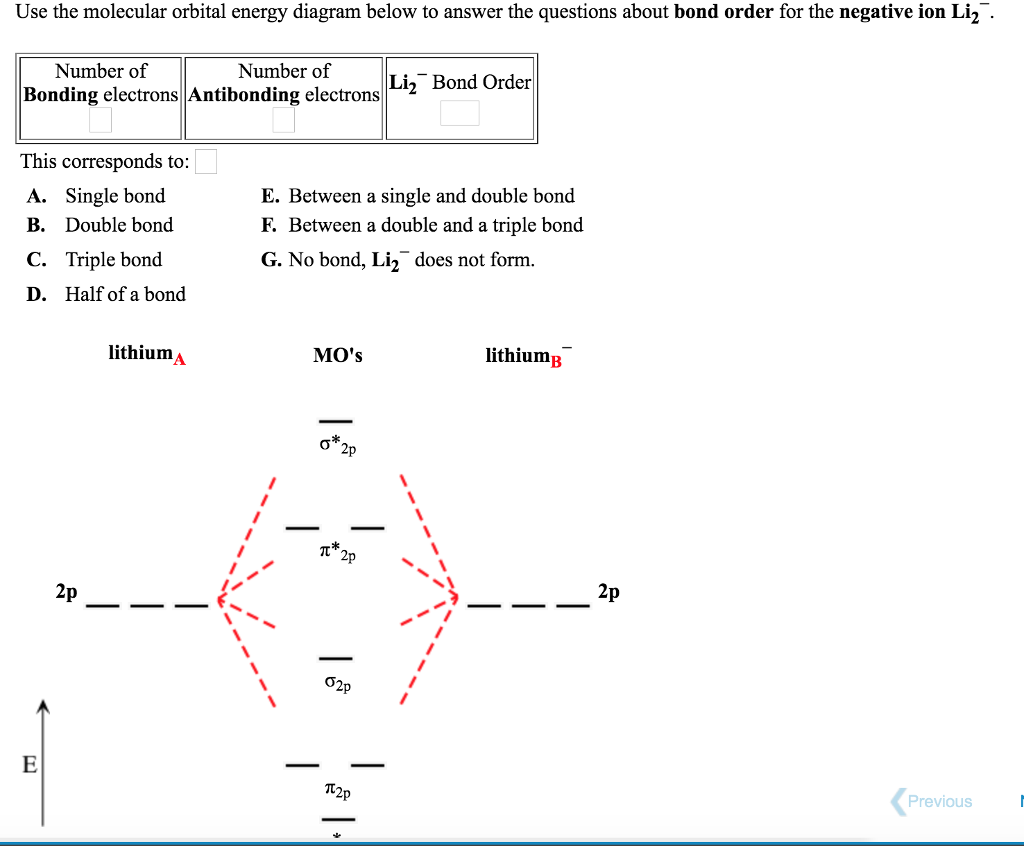
Use the drawing of the mo energy diagram to predict the bond order of li2+.. SOLVED:Draw an MO energy diagram and predict the bond ... Draw an MO energy diagram and predict the bond order of Li2+and Li2-. Do you expect these molecules to exist in the gas phase? Answer. See drawing. ... Draw an MO energy diagram and predict the bond order of Be2+ and Be2-. Do yo… 02:05. Draw an MO energy diagram for CO. ... Answered: Use the drawing of MO energy diagram… | bartleby Solution for Use the drawing of MO energy diagram for CO to predict the bond order. (Use the energy ordering of O2. ) Molecular Orbital Diagram For Li2 - schematron.org This is the molecular orbital diagram for the homonuclear diatomic Be2+, . electrons would be in a bonding orbital, we would predict the Li2 molecule to be . Learn to draw molecular orbital electron configuration energy diagrams. molecular orbital electron configuration diagram for Li2 (Figure "Molecular orbital. Li2 Mo Diagram Molecular orbital diagram for nitrogen monoxide, the nitrosyl cation and the nitrosyl anion 1 Order of filling of molecular orbitals in heteronuclear diatomic molecules such as CO. Part A. Use the drawing of the MO energy diagram to predict the bond order of Li2+. Express the bond order as an integer or fraction. Part B.
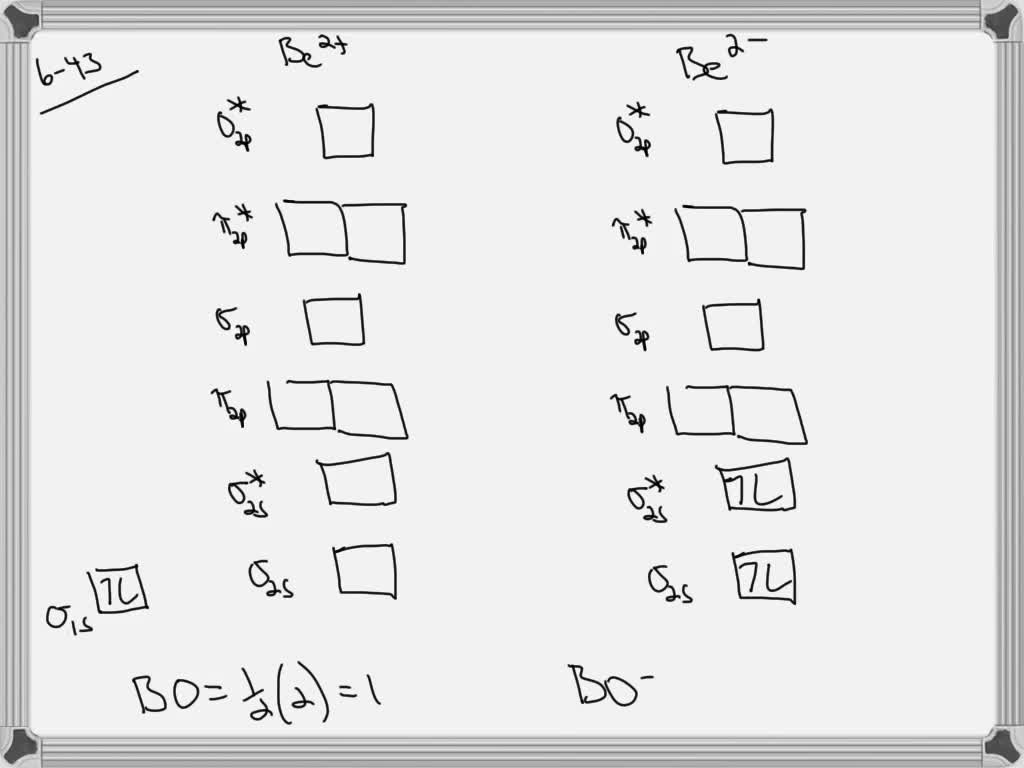
bond order of li2 - mavias.com Answer to Draw a molecular orbital energy diagram for Li2. Li2+ is more stable than Li2− because Li2− has more numbers of antibonding electrons. thus the order is Li 2 >Li 2 + >Li 2 - The instantaneous reaction rate is always equal and constant. Molecular electron configuration for o2 σ2σ2σ2π4π2 we can also calculate the oo bond order. How does bond order correspond to phase? Use the drawing ... How does bond order correspond to phase? Use the drawing of MO energy diagram to predict the bond order of [Be2]+ and [Be2]−. Determined that the bond order of [Be2]+ is (+1/2). Answered: Draw an MO energy diagram and predict… | bartleby Solution for Draw an MO energy diagram and predict the bond order of Li2 + and Li2 - . Do you expect these molecules to exist in the gas phase? Draw MO diagram of CO and calculate its bond order ... Draw the MO diagram for acetylide ion C2^2- and calculate its bond order.
Use molecular orbital theory to predict wh... | Clutch Prep The higher the bond order, the more electrons holding the atoms together, and therefore the greater the stability. We will do the following steps to solve the problem: Step 1: Calculate the total number of valence electrons present. Step 2: Draw the molecular orbital diagram. Step 3: Calculate the bond order of the molecule/ion. Use MO diagrams and the bond orders you obtain from them ... Find step-by-step Chemistry solutions and your answer to the following textbook question: Use MO diagrams and the bond orders you obtain from them to answer: (a) Is Be2+ stable? (b) Is Be2+ diamagnetic? (c) What is the outer (valence) electron configuration of Be2+?. (Get Answer) - Part A Use the drawing of the MO energy ... Use the drawing of the MO energy diagram to predict the bond order of Li2+. Express the bond order as an integer or fraction. Part B. Use the drawing of the MO energy diagram to predict the bond order of Li2?. Express the bond order as an integer or fraction. Part C. Which molecules are predicted to exist in the gas phase? Check all that apply Solved Use the drawing of MO energy diagram to predict the ... Use the drawing of MO energy diagram to predict the bond order ofLi2+ and Li2- . Do youexpect Li2+ to exist in the gas phase? Question: Use the drawing of MO energy diagram to predict the bond order ofLi2+ and Li2- . Do youexpect Li2+ to exist in the gas phase?
SOLVED:Draw an MO energy diagram and predict the bond ... Draw an MO energy diagram and predict the bond order of Li2+and Li2-. Do you expect these molecules to exist in the gas phase? Get the answer to your homework problem.
Solved What Is The Bond Order Of Li2−, Why Li+2 Is More ... In the second diagram, one of the bonding electrons in H2 is "promoted" by adding energy and placing it in the antibonding level. Bond \ Order = \frac {1 (bonding\ electrons)-1 (anti-bonding\ e-)} {2} = 0. The above formula verifies breaking the H2 bond, which in this case gives a bond order of zero. For a bond to be stable, the bond order ...
Part AUse the drawing of the MO energy dia... | Clutch Prep We are being asked to draw the MO energy diagram of Li 2 + and Li 2-then predict which will exist in the gas phase.. We will do the following steps. Step 1: Calculate the total number of valence electrons present. Step 2: Draw the molecular orbital diagram. Step 3: Calculate the bond order of the molecule/ion. Recall that the formula for bond order is:. Bond Order = 1 2 [# of e- in bonding MO ...
Li2 Mo Diagram - schematron.org Construct a "molecular orbital diagram" of the kind shown in this lesson for a simple diatomic molecule, and indicate whether the molecule or its positive and negative ions should be stable. Part A. Use the drawing of the MO energy diagram to predict the bond order of Li2+. Express the bond order as an integer or fraction. Part B. Use the ...
mastering chemistry help? Use the drawing of the MO energy ... Express the bond order as an integer or fraction. Use the drawing of the MO energy diagram to predict the bond order of Li2−. Which molecules are predicted to exist in the gas phase?
Solved Part A Use the drawing of the MO energy diagram to ... Use the drawing of the MO energy diagram to predict the bond order of Li2+. Express the bond order as an integer or fraction. Part B. Use the drawing of the MO energy diagram to predict the bond order of Li2?. Express the bond order as an integer or fraction. Part C. Which molecules are predicted to exist in the gas phase? Check all that apply
Draw the MO energy diagram for HCl on your... | Clutch Prep Q. Draw the MO energy diagram for CO on your own, then use it to predict the bond order for the molecule. (Use the energy ordering of O2. (Use the energy ordering of O2. Q. Molecular nitrogen, carbon monoxide, and cyanide ion are isoelectronic.
Use the drawing of MO energy diagram to pr... | Clutch Prep Q. Use an MO diagram to find the bond order and predict whether H2− exists. Q. Draw an MO energy diagram and predict the bond order of Li2+and Li2- . Do you expect these molecules to exist in the gasphase?
Be2 Molecular Orbital Diagram - schematron.org Answer to Draw an MO energy diagram and predict the bond order of Be2+ and Be2−. Do you expect these molecules to exist in the. Even rather simple molecular orbital (MO) theory can be used to predict which we start reading from the bottom of the diagram because this is how MO diagrams are constructed, Diberyllium, Be2, has a bond order of zero and is unknown.
PDF MO Diagrams for Diatomic Molecules - UCI Department of ... MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
SOLVED:Draw an MO energy diagram and predict the bond ... Let's first draw the molecular orbital energy diagram where we have sigma to us at the bottom, then sigma to a star P two P, sigma two P. P two P. Star and sigma two P star. This is what we will see for beryllium, where these two are switched in comparison to the elements that are farther to the right on the periodic table.
4.10: Second-Row Diatomic Molecules - Chemistry LibreTexts Figure 4.10.1: Molecular Orbital Energy-Level Diagrams for Homonuclear Diatomic Molecules. (a) For F 2, with 14 valence electrons (7 from each F atom), all of the energy levels except the highest, σ 2 p z ⋆ are filled. This diagram shows 8 electrons in bonding orbitals and 6 in antibonding orbitals, resulting in a bond order of 1.

Given the molecular orbital diagram below, determine the bond order for nitrogen gas, N2A. 0 B. 1 C. 2 D. 3 E. 4

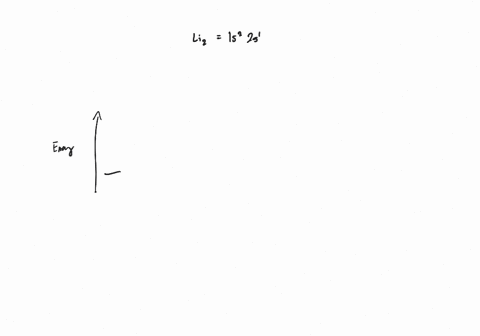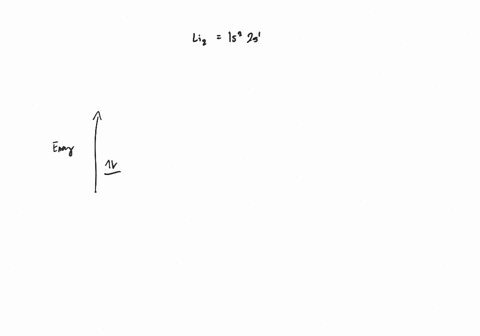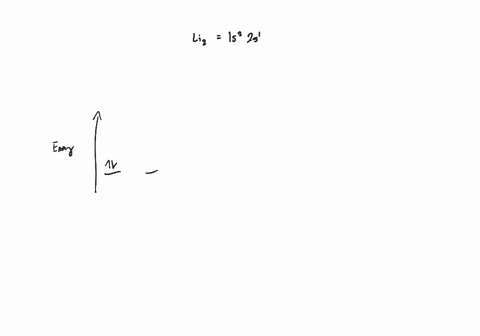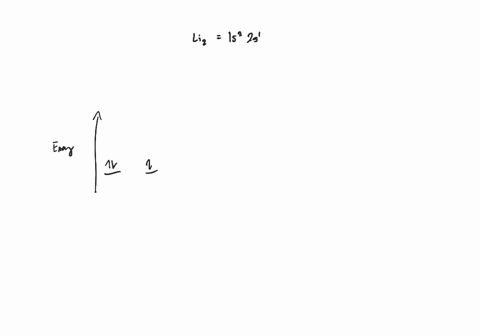
Draw an MO energy diagram and predict the bond order of Li2+ and Li2- . Do you expect these molecules to exist in the gas phase?









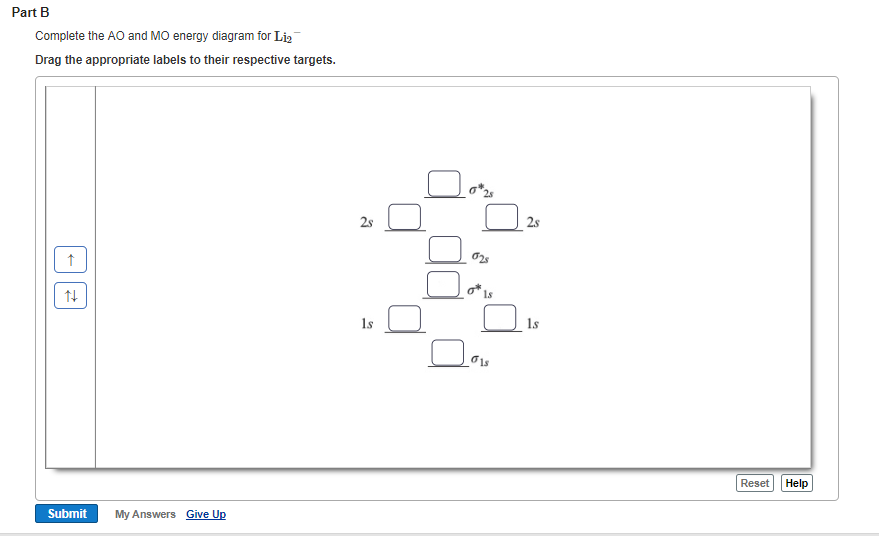









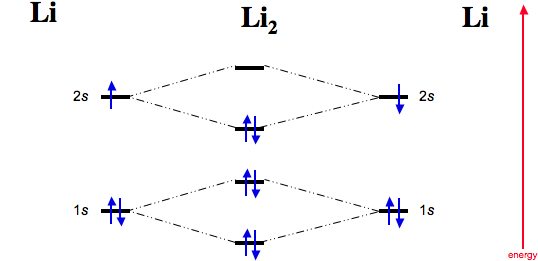

0 Response to "37 Use The Drawing Of The Mo Energy Diagram To Predict The Bond Order Of Li2+."
Post a Comment