40 d orbital energy level diagram
Molecular orbitals energy level diagram - Big Chemical ... Draw simple molecular orbital energy-level diagrams to indicate how the bonding in the saline hydrides, such as NaH or KH, differs from that between hydrogen and a light p-block element such as carbon or nitrogen. For each of the following, draw a molecular orbital energy level diagram and give the bond order. Tell whether the species would be more or less stable after gaining an electron, (a) 02+ (b) CN (c) S2 (d) NO (e) Be2+. 11.4: The Effect of the Metal Ion on d-Orbital Splitting ... The metal's electronic energy levels are shown on the other side. The result of their interaction, a metal-ligand complex, is shown in the middle. The d orbital splitting diagram is shown in a box. Suppose the diagram above is for a first row transition metal. The diagram for a second or third row metal is similar, but with stronger bonds.
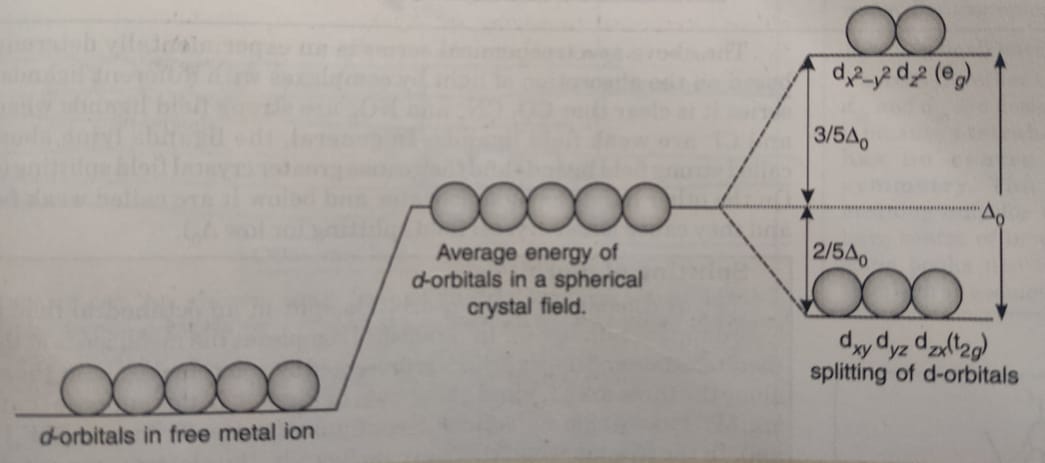
PDF Effect of Distortion on the d-Orbital Energy Levels Figure 46. The splitting pattern of d-orbital energy levels of d3-metal complexes in tetragonal distortion. 2. Rhombic distortion: The unequal amount of elongation or compression along two four-fold axes of rotation in octahedral complexes produces rhombic distortions. The common examples of rhombic distortion are high-

D orbital energy level diagram
s,p,d,f Orbitals - Chemistry | Socratic All levels except the first have p orbitals. d ORBITALS. In addition to s and p orbitals, there are two other sets of orbitals which become available for electrons to inhabit at higher energy levels. At the third level, there is a set of five d orbitals (with complicated shapes and names) as well as the 3s and 3p orbitals (3px, 3py, 3pz). At the third level there are a total of nine orbitals altogether. The five 3d orbitals are called PDF Metal d orbitals in an Oh crystal field greater the energy of the orbital. • Thus, thecoulbilombiccontib titribution to thepaiiiring energy tdtends to fllfall off in theorder 3d > 4d > 5d, asthe orbitalsbecome largerandthe electron interactions are lessened. • A high‐spin configuration avoids pairing by spreading the electrons across both the t 2g and e g levels. S P D F orbitals Explained - 4 Quantum Numbers, Electron ... This video explains s, p, d, and f orbitals, sublevels, and their shapes. It discusses the 4 quantum numbers n, l, ml, and ms. n represents the energy leve...
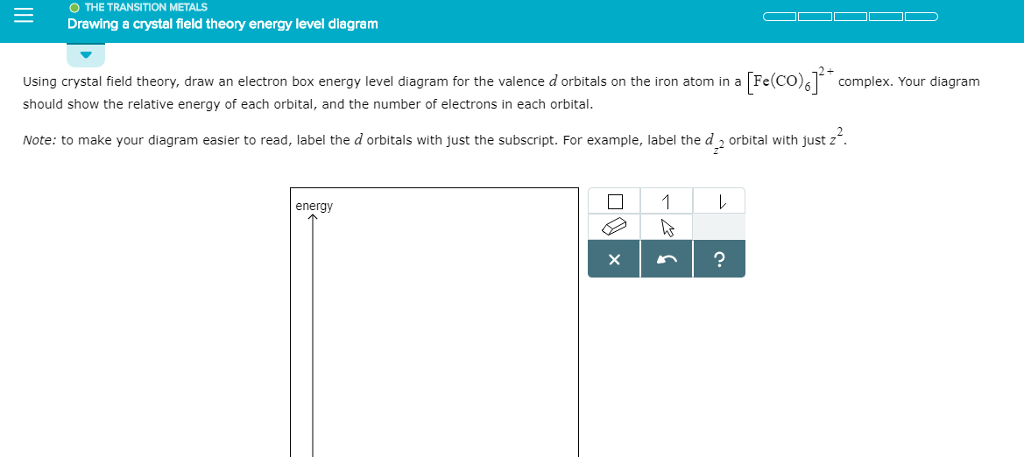
D orbital energy level diagram. PDF π-Bonding and Molecular Orbital Theory - Dalal Institute The electron previously filled in d-orbital of the free metal ion can now be considered as distributed between t2g * and e g *. Hence, the crystal field splitting Δ o decreases when ligand to metal bonding takes place. The overall molecular orbital energy level diagram for this type of π-bonding in octahedral complexes can be shown as: SOLVED:Draw an orbital energy-level diagram (like those in ... Problem 47 Easy Difficulty. Draw an orbital energy-level diagram (like those in Figs. 16.27 and 16.29 ) showing the configuration of d-electrons on the metal ion in each of the following complexes: ( a) [ C o ( N H 3) 6] 3 +; (b) [ N i C l 4] 2 − ( tetrahedral ); (c) [ F e ( O H 2) 6] 3 +; (d) [ F e ( C N) 6] 3 −. SOLVED:Sketch the d -orbital energy level diagrams for ... So the lower energy or bills are always filled first. And here we see that we have one unfairly electron. However, it in the other complex water is a relatively weak field splitting Lagan, so the electron, the energies of the orbital's are relatively similar. So as a result of hans rule, electrons are essentially added to each of the orbital's. 43 molecular orbital diagram h2 - Modern Wiring Diagram 39 h2+ molecular orbital diagram - Diagram Online Source 39 h2+ molecular orbital diagram. In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule.This function can be used to calculate chemical and physical properties such as the probability of finding an electron ...
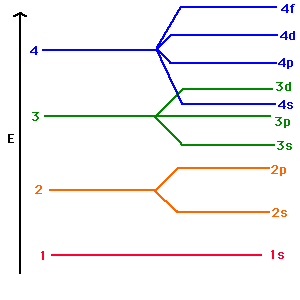
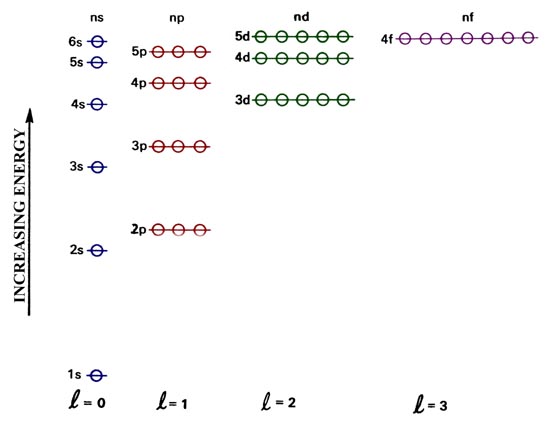
Energy Of Orbitals: Concept, Factors, Observations, and ... Energy level diagrams are diagrams that show the arrangement of orbitals in order of increasing energy. The principal quantum number alone can indicate the energy of an electron in a single atom. In multi-electron atoms, an electron's energy is determined by both its principal quantum number (n) and its azimuthal quantum number (l). Quantum Number Orbital - Definition, Formula, Diagram, Shape Quantum numbers or principal, azimuthal, magnetic, and spin quantum number and fine structure of electromagnetic spectrum lines of atoms in mechanics define the electron energy levels and shapes or diagram of s, p, d- orbital in physics and chemistry. Bohr's theory of hydrogen spectrum and Sommerfeld theory met with number of difficulties when these quantum theories applied to characteristics of poly-electronic atoms. Chlorine Orbital diagram, Electron configuration, and ... Orbital diagram:-A orbital diagram is simply a pictorial representation of the arrangement of electrons in the orbital of an atom, it shows the electrons in the form of arrows, also, indicates the spin of electrons.Electron configuration:- Electron configuration is the arrangement of electrons in atomic orbitals.It shows the electrons in numbers, It doesn't show the details on the spin of ... 1.Identify which of the following orbital | Chegg.com Science. Chemistry. Chemistry questions and answers. 1.Identify which of the following orbital designations do not exist: 1s, 7d, 4f, 3d 2.Draw the energy-level diagram for the following element: boron. 3.Write full and shorthand electronic configuration of: S and Fe2+.
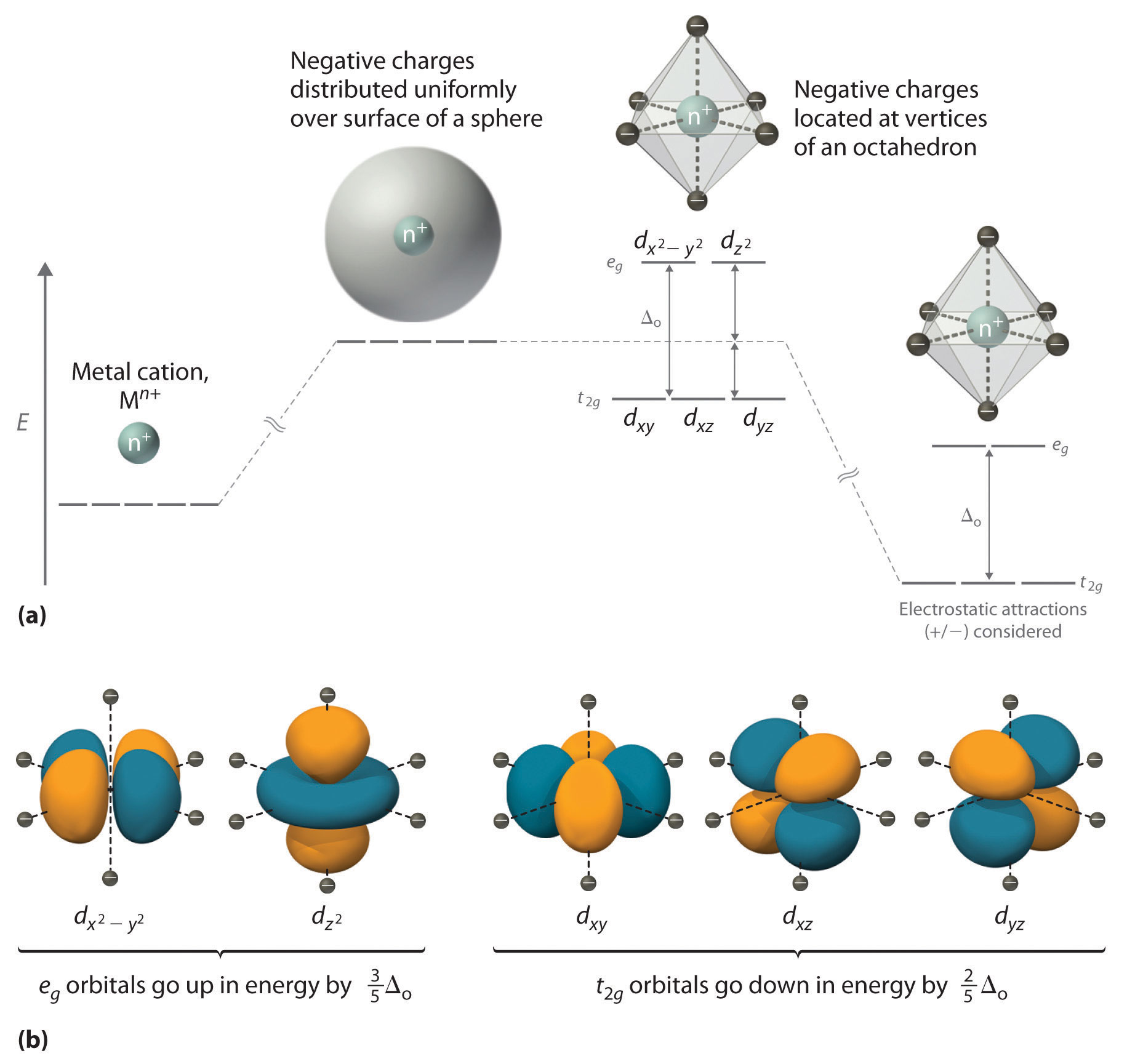
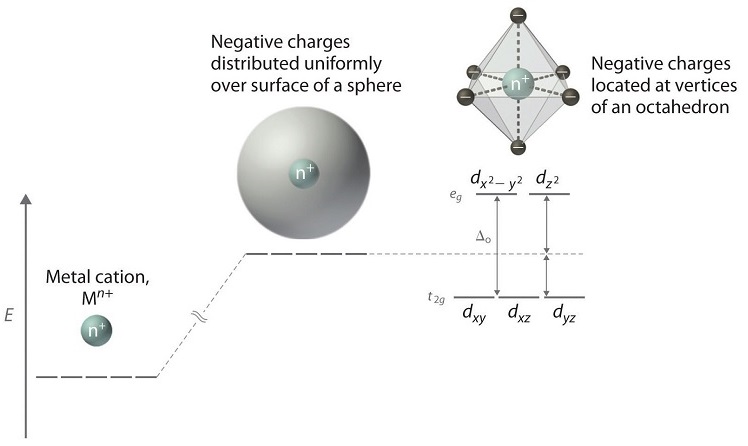
4.1.2: Introduction to Ligand Field ... - Chemistry LibreTexts If we translate that idea into a picture of the d orbital energy levels in an octahedral geometry, it looks like this: Figure \(\PageIndex{14}\): When the charge on the metal ion is increased, both the higher and the lower levels drop in energy. However, the lower level drops more. Thus, the gap between the levels gets wider. How to determine relative energy levels of d-orbitals ... How to determine relative energy levels of d-orbitals (Crystal Field Theory) Say you have an octahedral metal ion. Any textbook will tell you that the d-orbitals will split as this image shows. Every textbook I've read stops short of saying how this is calculated. Usually the explanation is "just look at it, lol" In simple geometries such as ... Solved Sketch the d-orbital energy level diagrams | Chegg.com Question: Sketch the d-orbital energy level diagrams for [Fe(OH2)6]3+ and [Fe(CN)6]3-. Use your diagrams to answer the questions. 1 a). Use your diagrams to answer the questions. 1 a). The aqua complex is i) low ii) high spin? Chapter 2.5: Atomic Orbitals and Their Energies ... Figure 2.5.10 Orbital Energy Level Diagram for a Typical Multielectron Atom. Because of the effects of shielding and the different radial distributions of orbitals with the same value of n but different values of l, the different subshells are not degenerate in a multielectron atom.
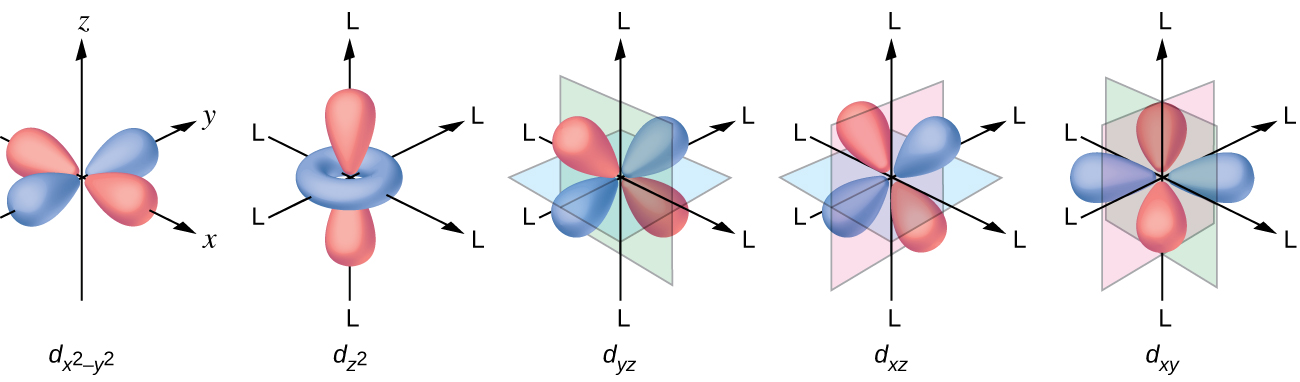
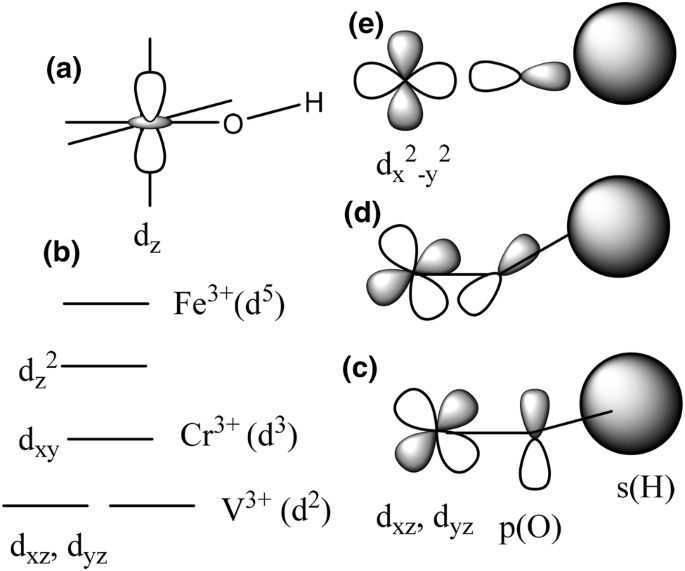
complex ions - more about d orbitals - chemguide But two of the d orbitals have lobes pointing along those axes - the 3d x 2 - y 2 and 3d z 2 orbitals. Those will feel more repulsion than the other three, which have lobes in between the axes. That means that two of the d orbitals will now have a higher energy than the other three - which is exactly what the diagram we have been using shows.
Degenerate Orbitals - Explanation With Diagram, Examples ... Explanation of Degenerate Orbitals with Diagram. Orbitals in the 2p sublevel are degenerate orbitals - Which means that the 2p x, 2p y, and 2p z orbitals have the exact same energy, as illustrated in the diagram provided below. Similarly, the 3p x, 3p y, and 3p z are degenerate orbitals. And at the 3d energy level, the 3d xy, 3d xz, 3d yz, 3d x2 - y2, and 3dz 2 are degenerate orbitals with the same energy.
PDF Molecular Orbital Theory - Octahedral, Tetrahedral or ... The overall molecular orbital energy level diagram for σ-bonding in octahedral complexes can be shown as: Figure 10. The formation of σ-molecular orbitals (bonding, antibonding and non-bonding) in octahedral complexes of transition metals. Buy the complete book with TOC navigation,
6.6: 3D Representation of Orbitals - Chemistry LibreTexts In three of the d orbitals, the lobes of electron density are oriented between the x and y, x and z, and y and z planes; these orbitals are referred to as the 3 d x y, \)3d_ {xz}\), and 3 d y z orbitals, respectively. A fourth d orbital has lobes lying along the x and y axes; this is the 3 d x 2 − y 2 orbital.
Orbitals - Orbital Energy & Orbital energy level - BYJUS Orbitals - Orbital Energy & Orbital energy level. The energy of an electron in a single atom can be determined solely by the principal quantum number. Orbitals can be ranked in the increasing order of orbital energy as follows: 1s < 2s = 2p < 3s = 3p = 3d <4s = 4p = 4d= 4f. However, the energy of an electron in multi-electron atoms depends on ...
PDF D-orbital splitting diagrams - University of California ... D-orbital splitting diagrams Use crystal field theory to generate splitting diagrams of the d-orbitals for metal complexes with the following coordination patterns: 1. Octahedral 2. Tetrahedral 3. Trigonal bipyramidal 4. Square pyramidal d z2x2-y d xy d yzxz 5. Square planar d z2x2-y d xy d yzxz d z2 d x2-yxy d yz d xz d z2 d x2-y2 d xy d yz d xz d z2 d x2-y d xy d yz d xz z x y
S P D F orbitals Explained - 4 Quantum Numbers, Electron ... This video explains s, p, d, and f orbitals, sublevels, and their shapes. It discusses the 4 quantum numbers n, l, ml, and ms. n represents the energy leve...
PDF Metal d orbitals in an Oh crystal field greater the energy of the orbital. • Thus, thecoulbilombiccontib titribution to thepaiiiring energy tdtends to fllfall off in theorder 3d > 4d > 5d, asthe orbitalsbecome largerandthe electron interactions are lessened. • A high‐spin configuration avoids pairing by spreading the electrons across both the t 2g and e g levels.
s,p,d,f Orbitals - Chemistry | Socratic All levels except the first have p orbitals. d ORBITALS. In addition to s and p orbitals, there are two other sets of orbitals which become available for electrons to inhabit at higher energy levels. At the third level, there is a set of five d orbitals (with complicated shapes and names) as well as the 3s and 3p orbitals (3px, 3py, 3pz). At the third level there are a total of nine orbitals altogether. The five 3d orbitals are called








![d-orbital energy levels in planar [MIIF4]2−, [MII(NH3)4]2+ ...](https://pubs.rsc.org/en/Content/Image/GA/D0DT02022B)














0 Response to "40 d orbital energy level diagram"
Post a Comment