41 orbital diagram for germanium
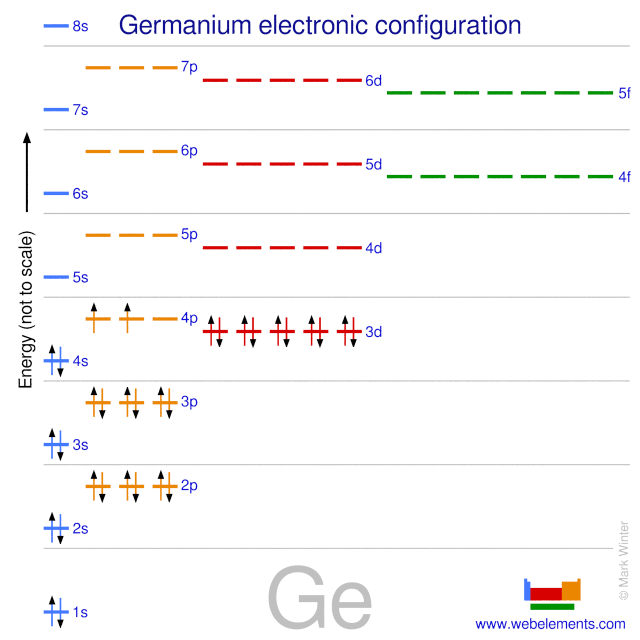
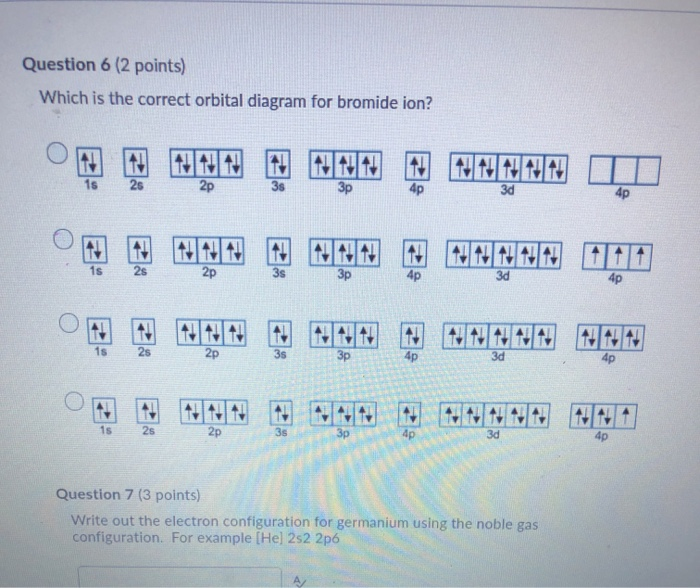
Write the orbital diagram for the ground state of the germanium atom. here we have to write Orbital Diagram for ground state Germany. The atomic number for Germanium is 32 here will follow this chart to how to Parents. The electrons in different shells. 1st 1 will be one is two electrons of opposite spain, one is two, then two is two. Germanium Orbital Diagram Electron Configurations and Orbital Diagrams KEY. Draw orbital diagrams for the following elements: 1. phosphorus. ↑↓. ↑↓ 4. germanium. ↑↓. Germanium atomic orbital and chemical bonding information. There are also tutorials on the first thirty-six elements of the periodic table.
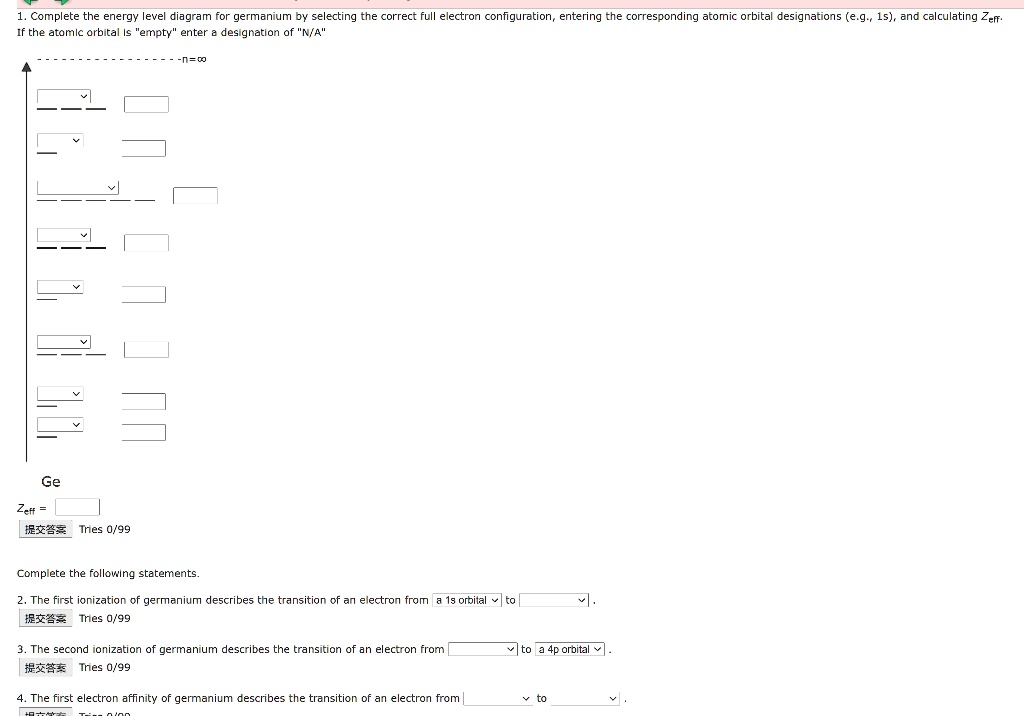
Chapter 7 Write an orbital diagram for silicon. SOLUTION Since silicon is atomic number 14, it has 14 electrons. Draw a box for each orbital, putting the lowest-energy orbital (1s) on the far left and proceeding to orbitals of higher energy to the right. The electron configuration of a germanium atom is.

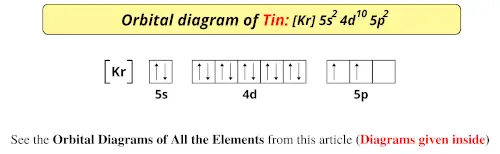
Orbital diagram for germanium
Sodium(Na) electron configuration and orbital diagram To create an orbital diagram of an atom, you first need to know Hund's principle and Pauli's exclusion principle. Hund's principle is that electrons in different orbitals with the same energy would be positioned in such a way that they could be in the unpaired state of maximum number and the spin of the... (PDF) Calculation of Wulff's diagram for germanium On Wulf's diagram the orientations form plateau in the vicinity of (110). The surfaces with such orientations An analysis of the orbital populations shows that indeed the nature of the hybridization of. The potential surface for unreconstructed {001} face of germanium has been calculated by the... Orbital Diagrams and Electron Configuration - Basic Introduction... This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram...
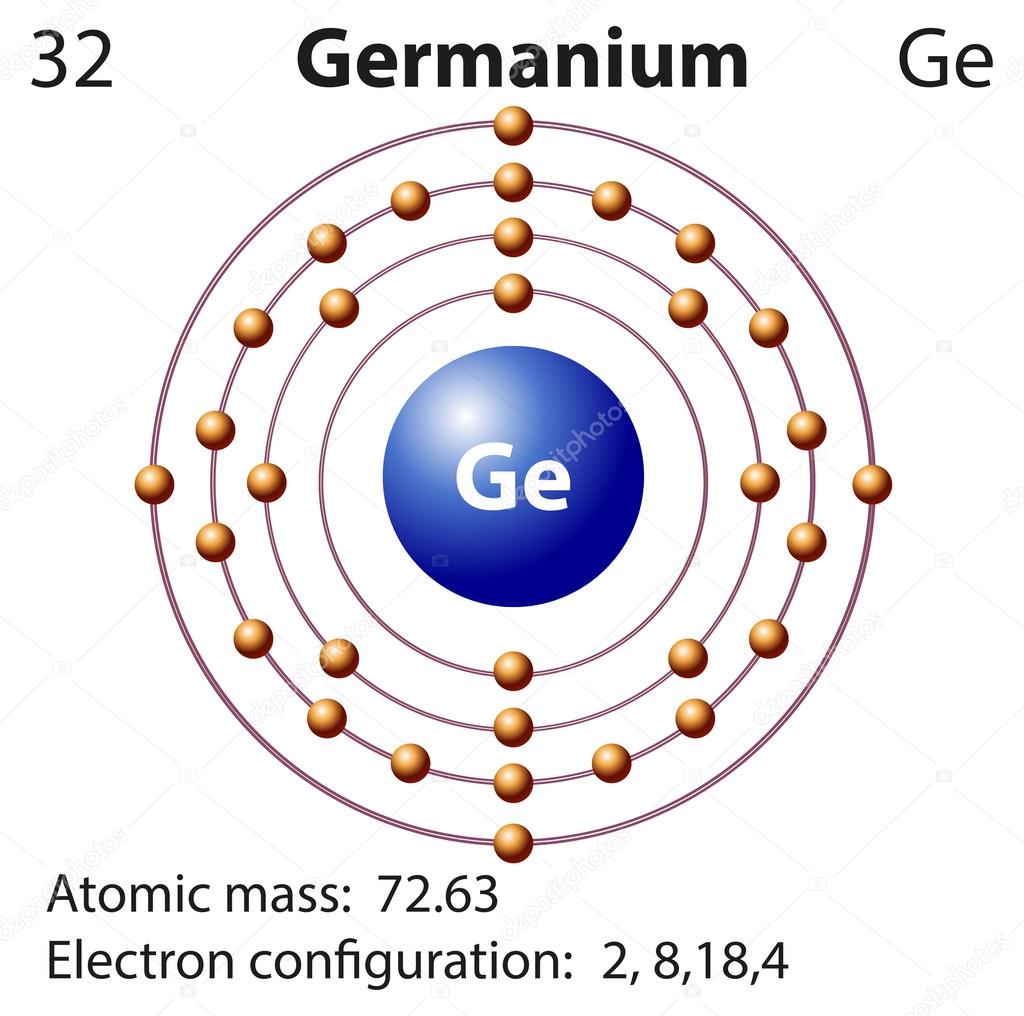
Orbital diagram for germanium. The Orbital Diagram For A Ground State Nitrogen Atom Is Choose The Orbital Diagram That Represents The Ground. Consider the bohr model of the hydrogen atom. A possible set of quantum numbers for the last electron added to complete an atom of germanium in its ground state is a. Since 1s can only hold two electrons the next 2 electrons for n... Chemistry Review (Orbital Hybridization) - T. Marquardt RCHS Science The following molecular orbital diagram may be used for problems 32-46. For oxygen and fluorine, the σ2p orbital should be 46. Assuming that the molecular orbital energy diagram for a homonuclear diatomic molecule applies to a Silicon and germanium are examples of this type of semiconductor. Lewis structure Oxygen Electron shell Molecular orbital diagram... Lewis structure Potassium Electron Diagram Argon, symbol, chemical Element, angle png. Electron configuration Germanium Electron shell Bohr model Valence electron, copper shell, chemical Element, electron png. Germanium (Ge) | Orbital Diagram Germanium (Ge) has an atomic mass of 32. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more.
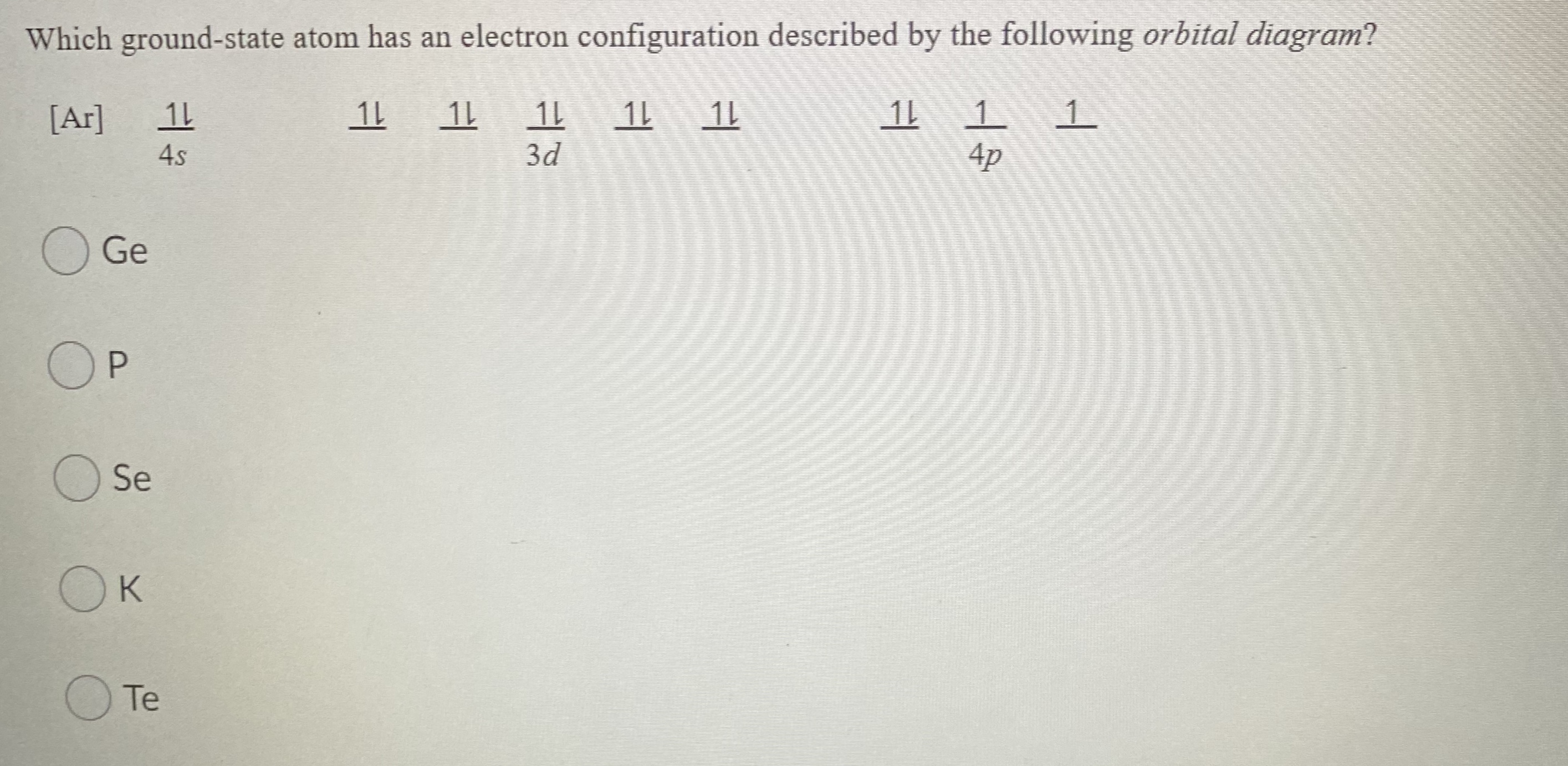
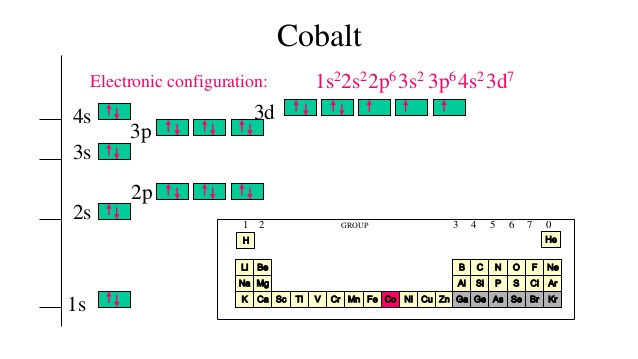
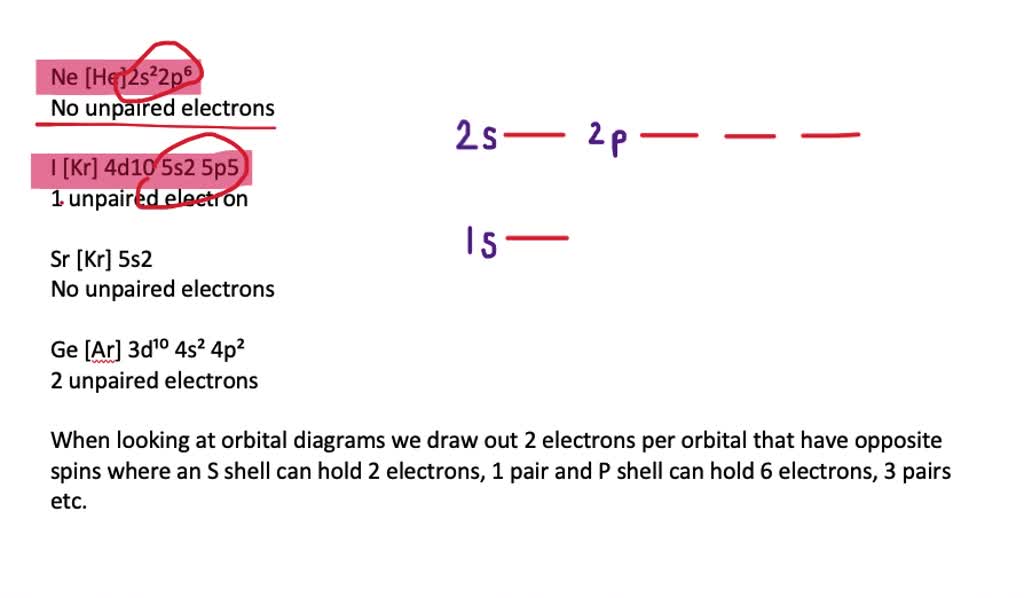
SOLUTION: calcul of frequency, and energy write the orbital diagram 9: Do the complete orbital diagram for germanium at the bottom of the page. (Abbreviated is fine.) a: Co b: Ca c: Cl d: W e: Hg f: Sn g: Ag h: Ar i: Am (element 95) 11: Do orbital diagrams for each of the following elements, then determine the number of unpaired electrons. Chapter 8: Periodic Properties of the Elements Flashcards | Quizlet ExamPLE 8.2 writing Orbital Diagrams Write an orbital diagram for sulfur and determine the number of unpaired electrons. The electron configuration of germanium written in order of increasing principal quantum number is: Ge1s22s22p63s23p63d104s24p2. Orbital Diagrams and Electron Configuration - Basic Introduction... This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram... (PDF) Calculation of Wulff's diagram for germanium On Wulf's diagram the orientations form plateau in the vicinity of (110). The surfaces with such orientations An analysis of the orbital populations shows that indeed the nature of the hybridization of. The potential surface for unreconstructed {001} face of germanium has been calculated by the...
Sodium(Na) electron configuration and orbital diagram To create an orbital diagram of an atom, you first need to know Hund's principle and Pauli's exclusion principle. Hund's principle is that electrons in different orbitals with the same energy would be positioned in such a way that they could be in the unpaired state of maximum number and the spin of the...






/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)

















![6] (a) Write an orbital diagram for the ground state of ...](https://img.homeworklib.com/images/b4358686-1dc6-4c4c-9ed3-6dd71a4b0aa1.png?x-oss-process=image/resize,w_560)
0 Response to "41 orbital diagram for germanium"
Post a Comment