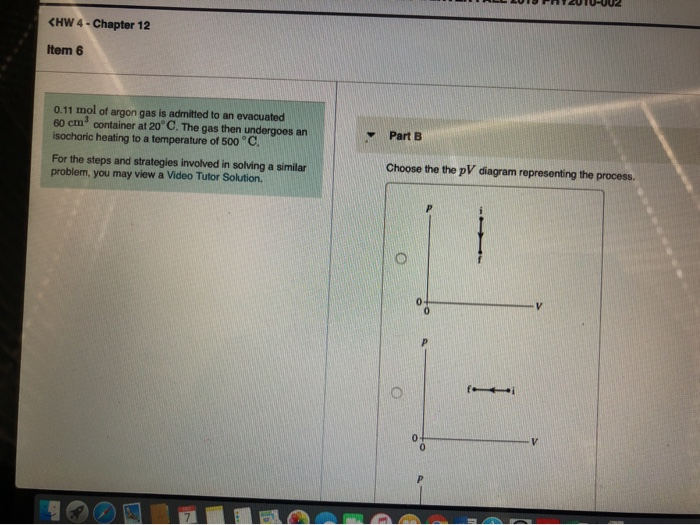
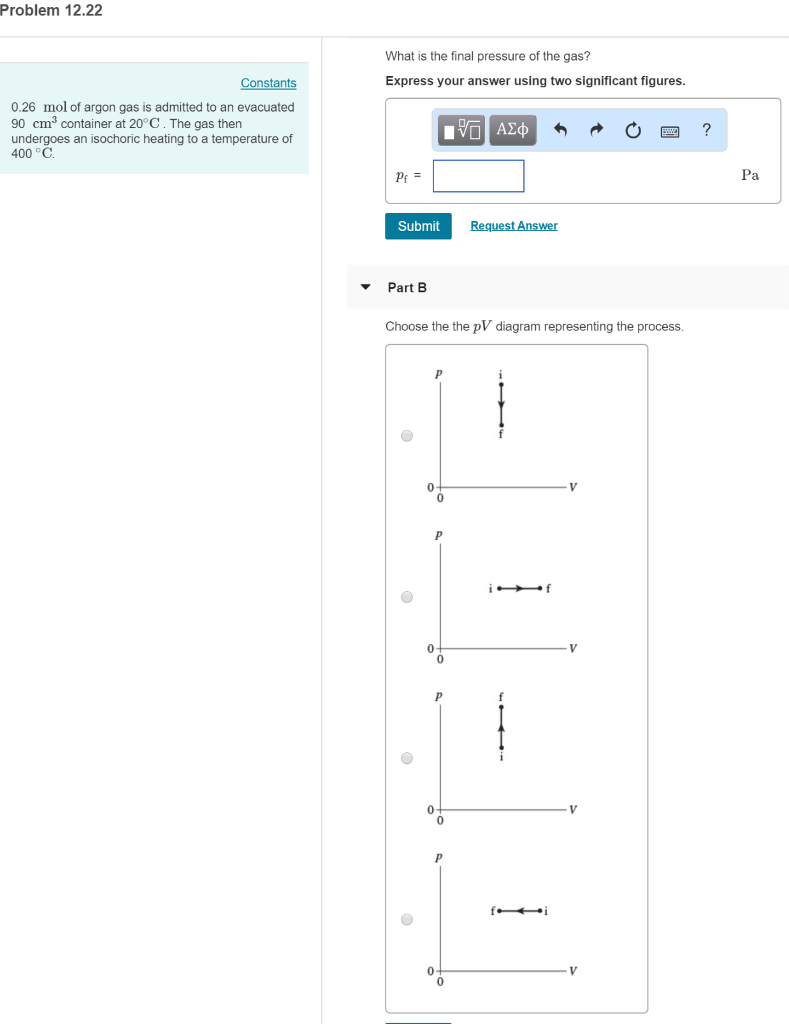
39 choose the the pv diagram representing the process.
m. 7 in decreases A PV diagram for a thermodynamical ... Then during process from A to B choose the m. 7 in decreases A PV diagram for a thermodynamical process is shown in the figure. A VO avo (1) Temperature continuously falls (2) Temperature first fallsiner rises (3) Temperature first rises then falls (4) Temperature continuously rises A PV diagram for a thermodynamical process is 2021/8/17 21:00 Solved 0.50 mol of argon gas is admitted to an ... - Chegg The gas then undergoes an isobaric heating to a temperature of 370 ∘C. What is the final volume of the gas? Choose the pV diagram representing the process. Question: 0.50 mol of argon gas is admitted to an evacuated 50 cm3 container at 20∘C. The gas then undergoes an isobaric heating to a temperature of 370 ∘C.
Solved 0.28 mol of argon gas is admitted to an ... - Chegg.com Choose the the pV diagram representing the process. Question: 0.28 mol of argon gas is admitted to an evacuated 40 cm3 container at 20∘C . The gas then undergoes an isochoric heating to a temperature of 300 ∘C∘ What is the final pressure of the gas? Choose the the pV diagram representing the process.

Choose the the pv diagram representing the process.
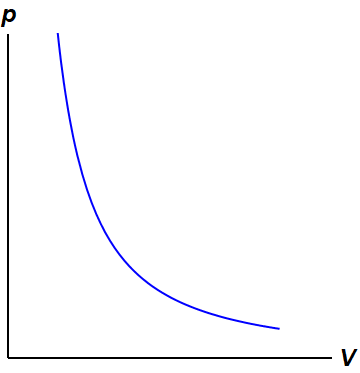
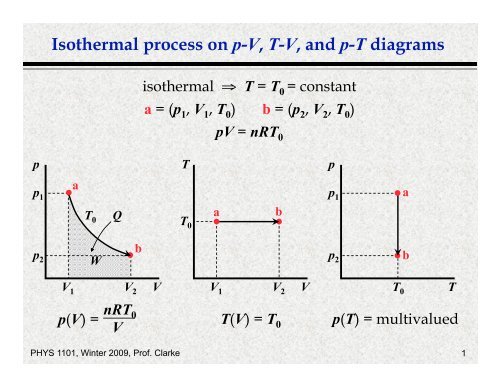
What is Isothermal Process - Definition On a p-V diagram, the process occurs along a line (called an isotherm) that has the equation p = constant / V. Boyle-Mariotte Law Boyle-Mariotte Law is one of the gas laws. At the end of the 17th century, Robert William Boyle and Edme Mariotte independently studied the relationship between the volume and pressure of a gas at constant temperature. [Solved] Figure shows the P-V diagram for a fixed mass of ... In P-V diagram: AB represents an isothermal process (hyperbolic). BC represents constant pressure process ( isobaric). AC represents constant volume process (isochoric). In P-T diagram AB will be a vertical straight line (isothermal process). BC will be a horizontal line (constant pressure process). In a PV diagram, how do you know the process is ... - Quora In a PV diagram, the process would be isothermal if the product of the value of the Pressure and the volume remains constant, PV=constant, whitherever the constant value is. The value of the constant will depend on the temperature, but it remains constant if the temperature remains constant.
Choose the the pv diagram representing the process.. PDF Thermodynamic Properties and calculation each process, Q and W are path-dependent. Since the work for each of these mechanically reversible processes is given by , the work for each process is proportional to the total area below the paths on the PV diagram representing the process. The relative sizes of these areas correspond to the numerical values of W. Q = ¿ 7 F9 = 0 F5,600 = F5,600J Ideal Gas Law | Boundless Physics - Lumen Learning For an ideal gas, from the ideal gas law PV = NkT, PV remains constant through an isothermal process. A curve in a P-V diagram generated by the equation PV = const is called an isotherm. For an isothermal, reversible process, the work done by the gas is equal to the area under the relevant pressure -volume isotherm. Which process is represented by the PV diagram? Isobaric process is a process where the pressure inside a system remains unchanged. In the Pressure Volume graph given, you can see that the pressure (y axis) remains constant with an increasing volume ( x axis). PV diagrams - part 2: Isothermal, isometric, adiabatic ... So on a PV diagram, an isothermal process is gonna look something like this, it's gonna curve like 1/x and it can be an isothermal expansion if volume increases or an isothermal compression if volume decreases. So the actual shape of the line drawn on a PV diagram for an isothermal process is sometimes called an isotherm and they look like that.
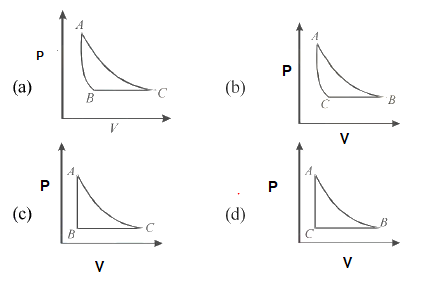
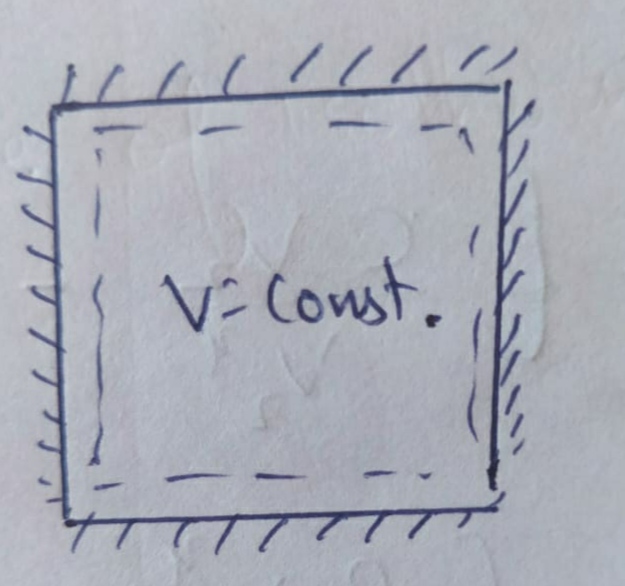
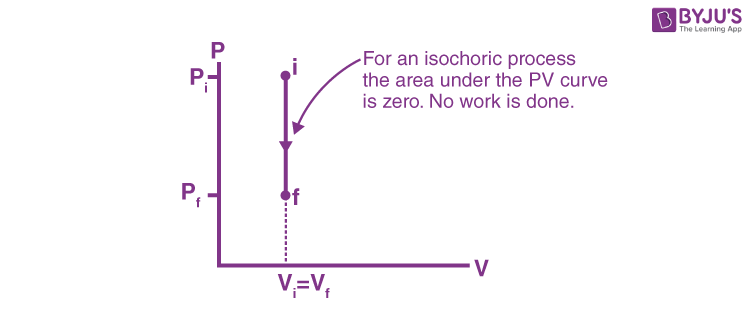
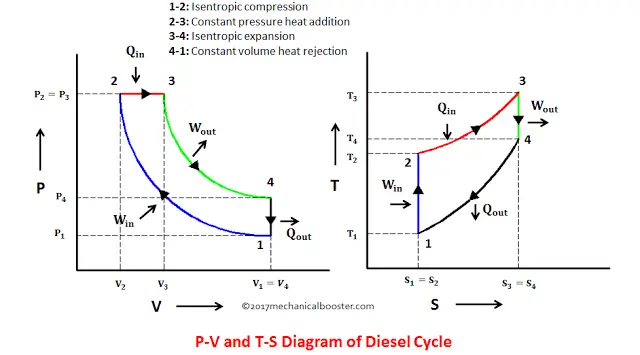
Pressure-Volume Diagrams - The Physics Hypertextbook Temperature is the slave of pressure and volume on a pressure-volume graph (PV graph). Function of State. ∆U = 3 2 nR∆T. Function of Path: Work. W = ∫ F · ds = ∫ P dV. W = − area on PV graph. Function of Path: Heat. Q = ∆U + W = nc∆T A CYCLINC PROCESS Figure 19.12 shows a pV-diagram for a ... IDENTIFY and SET UP: We must relate the change in internal energy, the heat added, and the work done in a thermodynamic process. Hence we can apply the first PDF Chapter 10 Refrigeration & Heat Pump Cycles (Systems) process (condenser and evaporator), the pressure of the refrigerant will be the saturation pressure during these process. Accordingly, (a) Low pressure => large specific volume refrigerant => large equipment (b) High pressure => smaller equipment, but must be designed to withstand higher pressure. Which of the following P - V diagram representing the ... Click here👆to get an answer to your question ️ Which of the following P - V diagram representing the graph of isomeric process? Join / Login > 11th > Physics > Thermodynamics > Thermodynamic Processes ... ISomeric process is equal to isochoric process. i.e, volume is constant.
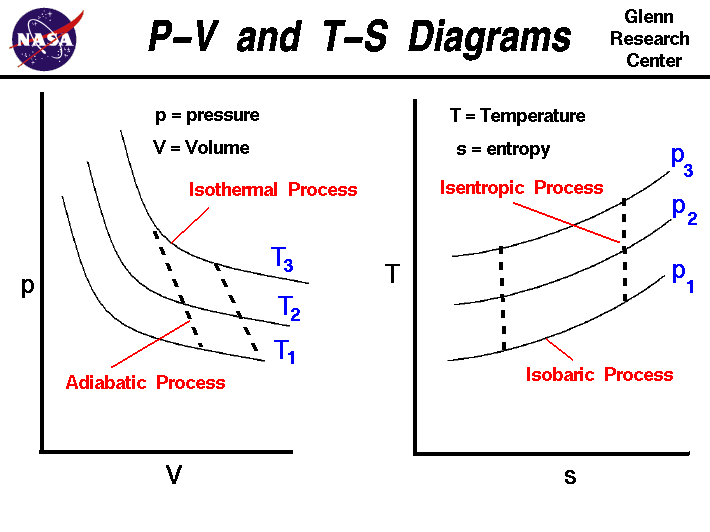
b In any cyclic process so Here that means that is 500 J ... EVALUATE: In cyclic processes, the total work is positive if the process goes clockwise around the pV-diagram representing the cycle, and neg-ative if the process goes counterclockwise (as here). Q = - 500 J; Q = W. ¢ U = 0, b S a a S b W = 1 V 2 V 1 pdV. V 2 6 V 1 Example 19.4 Comparing thermodynamic processes The pV-diagram of Fig. 19.13 ... Thermodynamic Processes & Their Types | Thermodynamic System Cyclic Process: It is a process in which the final state of the system is equal to the initial state. As we know, change in internal energy is a state function, so, in this case, ∆U = 0. Thermodynamics Process Examples. 1. Following is a PV curve showing two isothermal processes for two different temperatures. P-V and T-S Diagrams - NASA An isentropic process appears as a vertical line on a T-s diagram. The area under a process curve on a T-s diagram is related to the amount of heat transferred to the gas. It is possible to perform a series of processes, in which the state is changed during each process, but the gas eventually returns to its original state. What are PV diagrams? (article) - Khan Academy Every point on a PV diagram represents a different state for the gas (one for every possible volume and pressure). As a gas goes through a thermodynamics process, the state of the gas will shift around in the PV diagram, tracing out a path as it moves (as shown in the diagram below).
NCERT Exemplar Class 11 Physics Chapter 11 ... - Learn CBSE Out of the following diagrams, which figure represents the T-P diagram? i.e., V 1/p or PV = Constant. Hence, we can say that the gas is going through an isothermal process. Clearly, from the graph that between process 1 and 2 temperature is constant and the gas expands and pressure decreases, i.e., P 2
Which of the following P - V diagram representing the ... Click here👆to get an answer to your question ️ Which of the following P - V diagram representing the graph of isomeric process? Solve Study Textbooks. Join / Login >> Class 11 >> Physics >> Thermodynamics ... ISomeric process is equal to isochoric process. i.e, volume is constant.
T&HE: LESSON - 19 p-V DIAGRAM, T-s DIAGRAM, p-T DIAGRAM ... p-V diagram for water (solid-liquid-vapor region) If we heat ice at different vapor pressures and note down the corresponding change in volumes, the saturation state points for solid, liquid and vapor (state from which a change of phase may occur without change of pressure and temperature) for different pressures may be obtained on a p-V diagram.
PDF Isothermal process on p V T V, and p T diagrams ideal gas law: pV = nRT Consider the p-V diagram below in which the system evolves from a → b → c. If T 0 ~ 240K (and thus RT 0 = 2,000 J mol-1), how many moles of gas, n, are in the system? a) 5 b) 105 c) 50 d) 1,000 e) Not enough information to tell n = = = 50 pV RT 0 100,000 2,000 isotherm isobar isochor
Adiabatic Process - Definition, Equation, Reversible ... Reversible adiabatic process is also called an Isentropic Process. It is an idealized thermodynamic process that is adiabatic and in which the work transfers of the system are frictionless; there is no transfer of heat or of matter and the process is reversible. Such an idealized process is useful in engineering as a model of and basis of comparison for real processes.
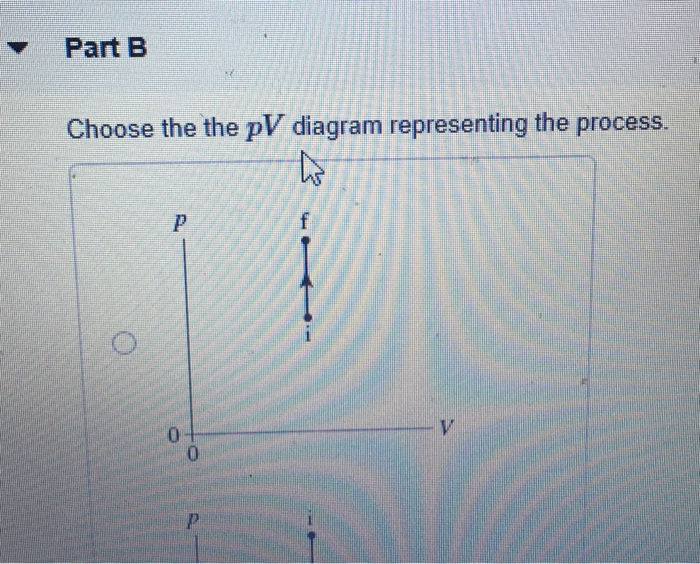
Solved 0.27 mol of argon gas is admitted to an ... - Chegg.com Express your answer in pascals. pf = nothing Pa Request Answer Part B Choose the the pV diagram representing the process. Question : 0.27 mol of argon gas is admitted to an evacuated 20 cm3 container at 20∘C.
PV diagram representing the internal expansion process ... Download scientific diagram | PV diagram representing the internal expansion process. from publication: Reciprocating Expander for an Exhaust Heat Recovery Rankine Cycle for a Passenger Car ...
Phase Diagrams - Chemistry - University of Hawaiʻi A phase diagram combines plots of pressure versus temperature for the liquid-gas, solid-liquid, and solid-gas phase-transition equilibria of a substance. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure dependence of the phase-transition temperatures (melting points, sublimation points, boiling points).
Prove that it is impossible for two lines representing ... Find step-by-step Chemistry solutions and your answer to the following textbook question: Prove that it is impossible for two lines representing reversible, adiabatic processes on a PV diagram to intersect..
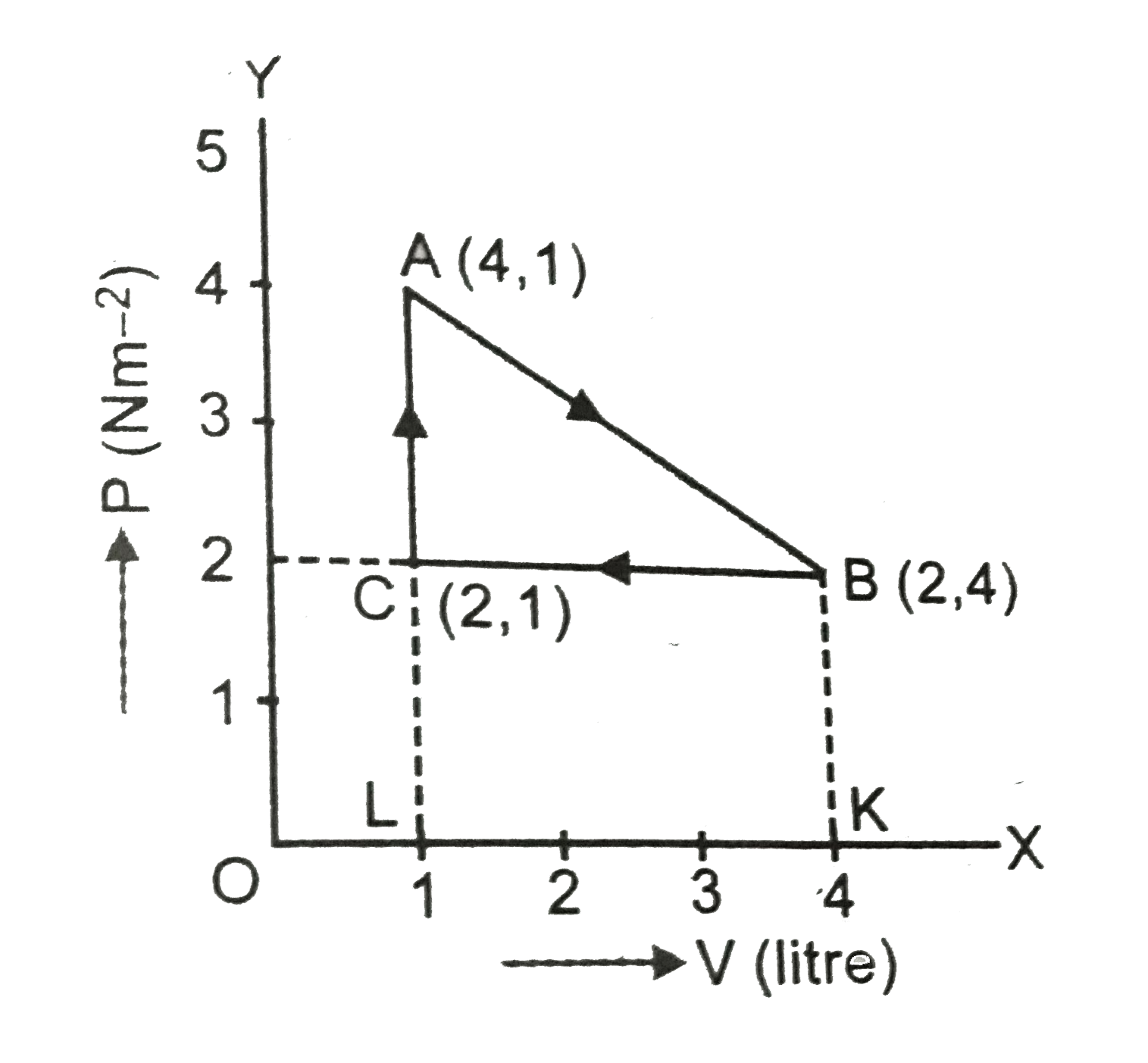
Diagrams of Thermodynamic Cycles — Collection of Solved ... The process is isothermal. This process is in the pV diagram represented by a hyperbole in such a way, so that the volume increases. The segment CA shows a constant pressure = an isobaric process, in the pV diagram represented by a horizontal line from the right to the left, because the volume decreases.
MasteringPhysics 2.0: Problem Print View - hi Hint F.1: Use the pV diagram to compare the work done: Since , work can be found by integrating the function with respect to volume (as you did in Part D). However, that integral also represents the area under the curve on the pV diagram. Compare the areas under the isobaric and isothermal curves to determine in which case more work is done.
In a PV diagram, how do you know the process is ... - Quora In a PV diagram, the process would be isothermal if the product of the value of the Pressure and the volume remains constant, PV=constant, whitherever the constant value is. The value of the constant will depend on the temperature, but it remains constant if the temperature remains constant.
[Solved] Figure shows the P-V diagram for a fixed mass of ... In P-V diagram: AB represents an isothermal process (hyperbolic). BC represents constant pressure process ( isobaric). AC represents constant volume process (isochoric). In P-T diagram AB will be a vertical straight line (isothermal process). BC will be a horizontal line (constant pressure process).
What is Isothermal Process - Definition On a p-V diagram, the process occurs along a line (called an isotherm) that has the equation p = constant / V. Boyle-Mariotte Law Boyle-Mariotte Law is one of the gas laws. At the end of the 17th century, Robert William Boyle and Edme Mariotte independently studied the relationship between the volume and pressure of a gas at constant temperature.
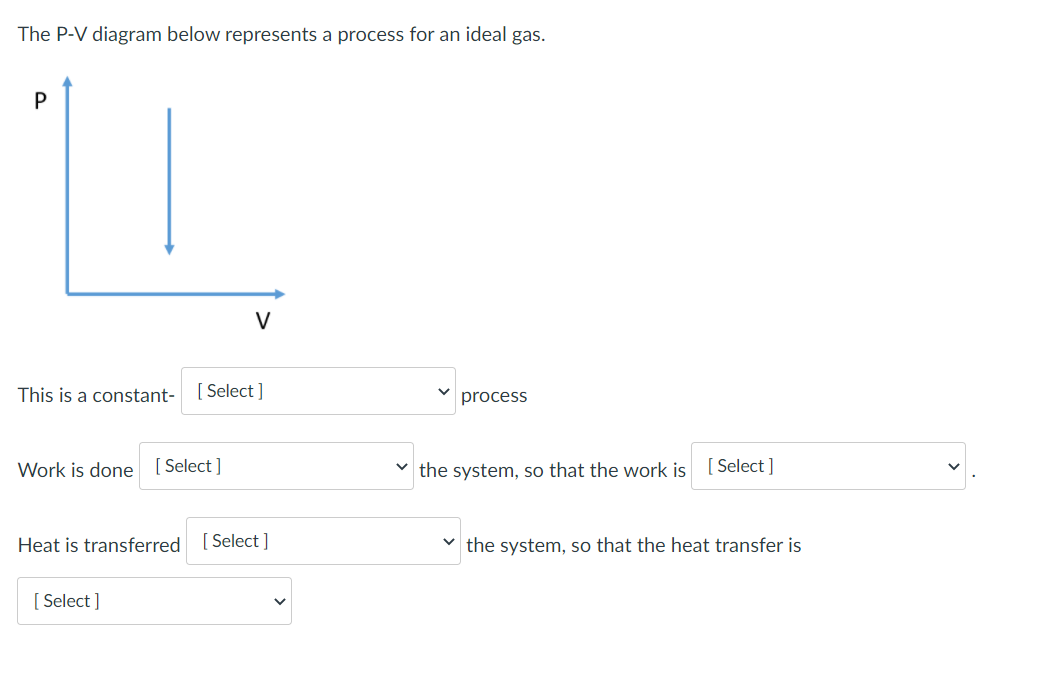
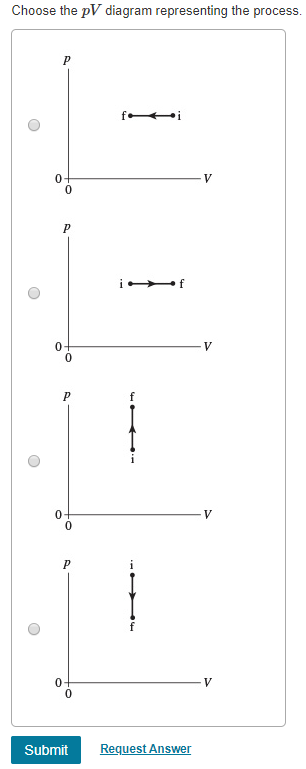


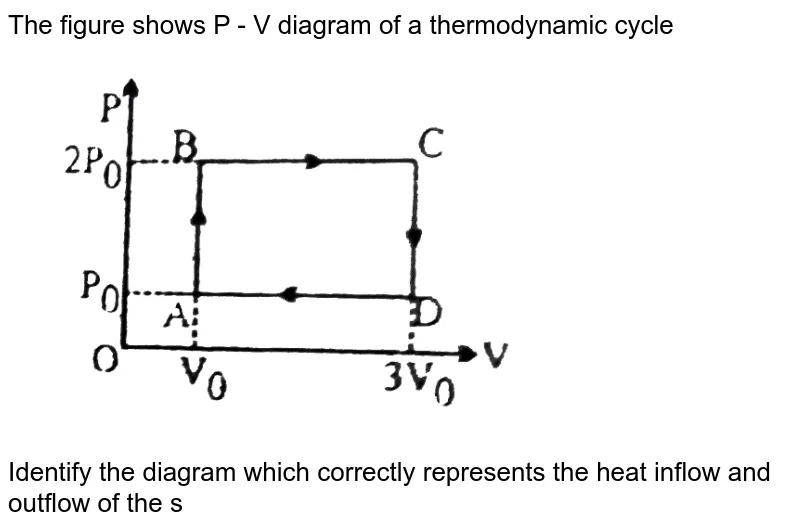








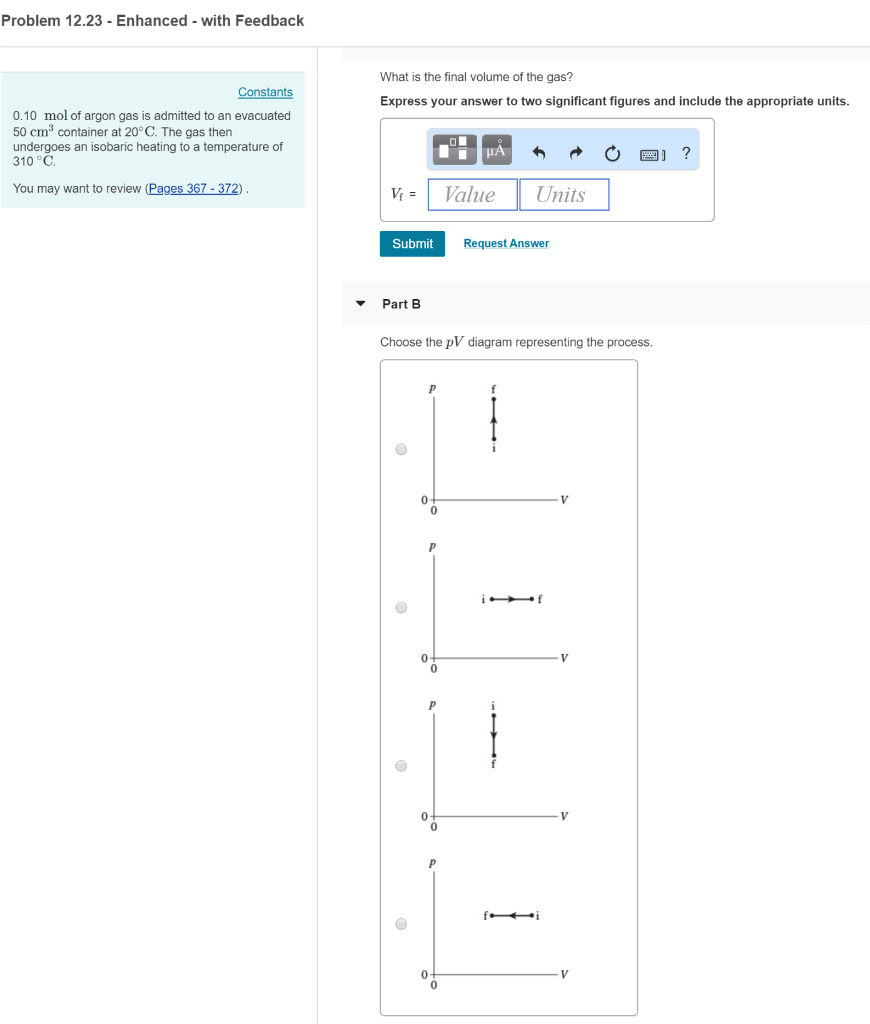
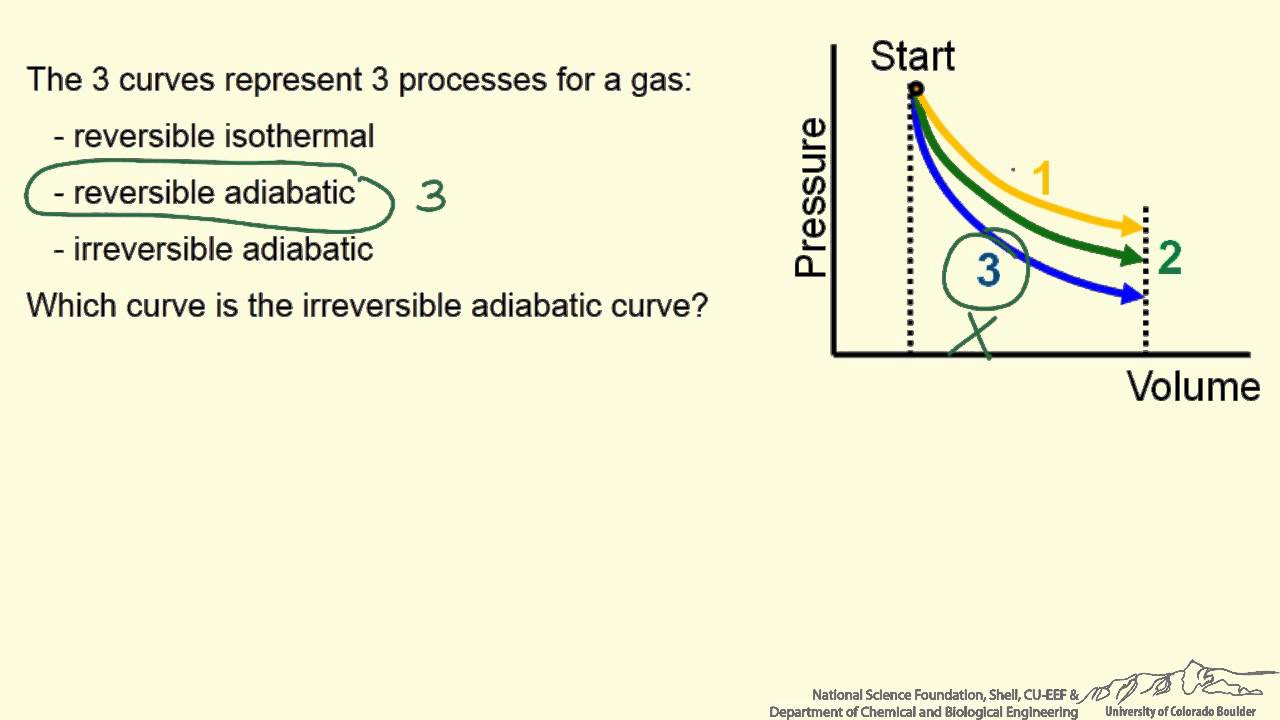
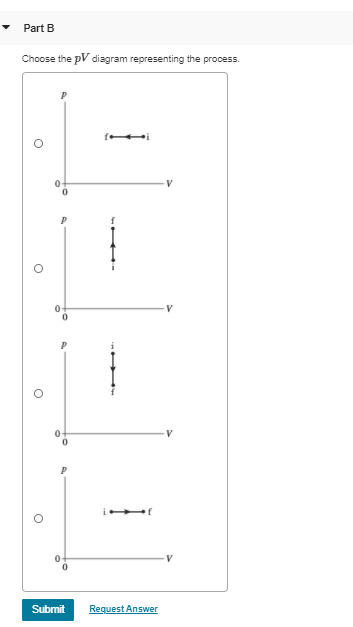










0 Response to "39 choose the the pv diagram representing the process."
Post a Comment