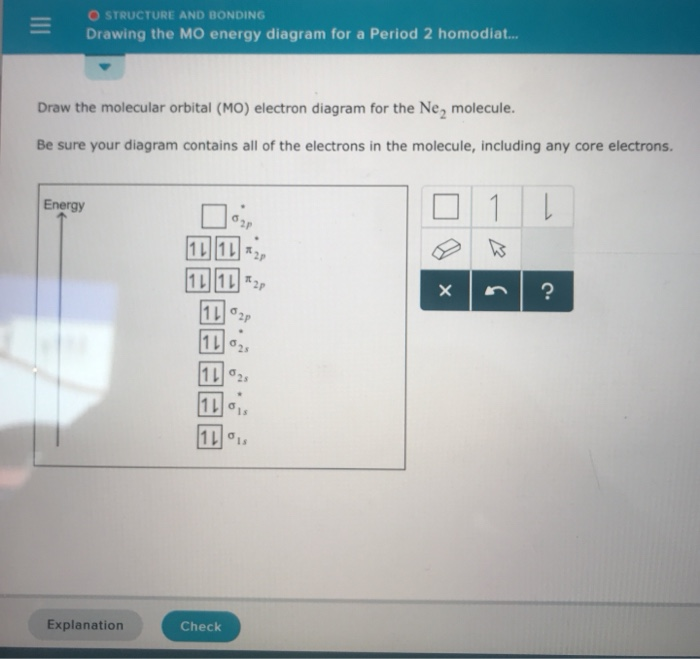
39 ne2 molecular orbital diagram
7.7 Molecular Orbital Theory - Chemistry Fundamentals The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram (Figure 7.7.9). For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right. Each horizontal line represents one orbital that can hold two electrons. Solved Draw The Valence Bond Lewis Structure of Ne2^+2 ... This problem has been solved! Draw The Valence Bond Lewis Structure of Ne2^+2. Draw Molecular Orbital Diagram using Shorthand Notation. What is the bond order, number of sigma bonds, number of pi bonds? Is it paramagnetic? Who are the experts? Experts are tested by Chegg as specialists in their subject area.
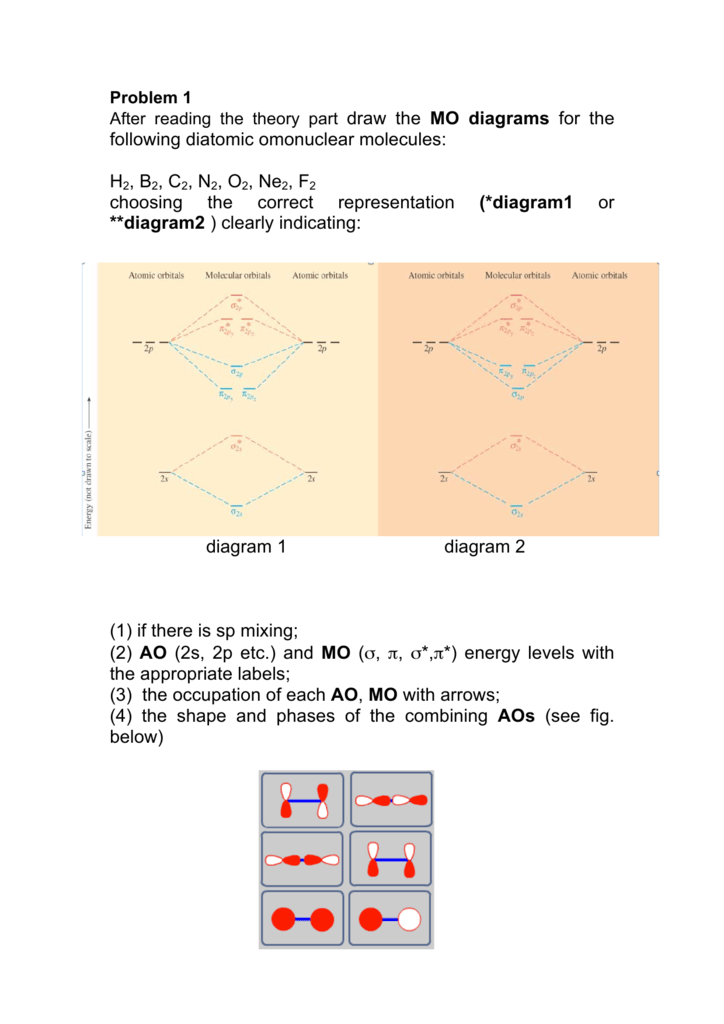
PDF Simple Molecular Orbital Theory - University of California ... Using Symmetry: Molecular Orbitals One approach to understanding the electronic structure of molecules is called Molecular Orbital Theory. • MO theory assumes that the valence electrons of the atoms within a molecule become the valence electrons of the entire molecule.
Ne2 molecular orbital diagram
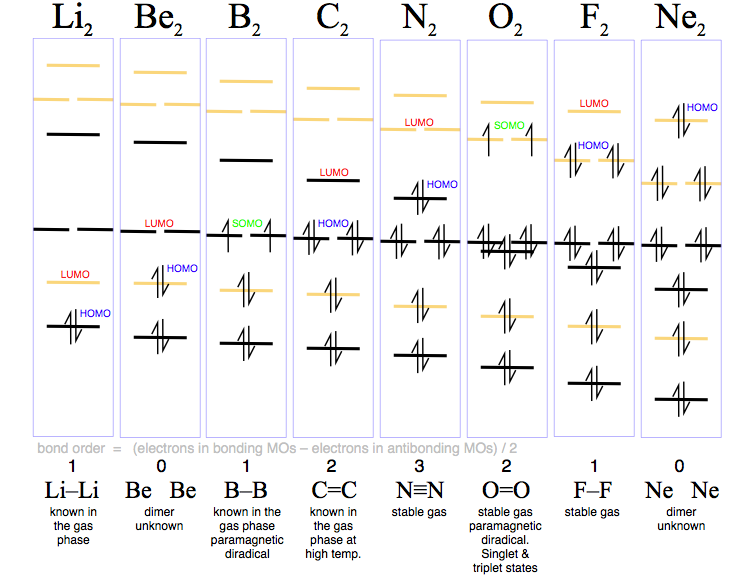
41 li2+ molecular orbital diagram - Wiring Diagrams Manual 7.7 Molecular Orbital Theory - Chemistry Fundamentals The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram (Figure 7.7.9). For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right. how to find bond order of ne2 - profjayaraman.com Molecular orbital diagram for ne2 . Bond order = [(number of bonding electon â number of antibonding electron)/2] Now, for N2â it is 2.5 See the MO diagram of N2â It is defined as the heat of formation for ions of opposite charge in the gas phase to combine into an ionic solid. The graphical representation presented in Fig. MO Diagram for N2+ (Molecular Orbital) - YouTube There are two MO diagrams you need to memorize for diatoms (N2, O2, Ne2, etc).One is for the elements up to Nitrogen. The other is for AFTER nitrogen (start...
Ne2 molecular orbital diagram. PDF MO Diagrams for Diatomic Molecules Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in Draw the molecular orbital diagram of N2N2 + N2 Write ... This picture shows the molecular orbital diagram of N 2. Orbitals represented by ∗ are antibonding orbitals and the orbitals without ∗ are bonding orbitals. Bond order can be calculated by the formula: Bond order = bonding electrons - antibonding electrons 2 electronic configuration - Molecular orbital (MO) diagram ... Show activity on this post. I have been taught that the MO diagram is different for molecules with 14 or less electrons than the one used for molecules with 15 or more electrons. For N X 2 the orbitals in increasing energy are: σ 1 s < σ 1 s ∗ < σ 2 s < σ 2 s ∗ < π 2 p x, π 2 p y < σ 2 p z < π 2 p x ∗, π 2 p y ∗ < σ 2 p z ∗ ... 37 use the molecular orbital diagram shown to determine ... chemistry 1a Chapter 10 Flashcards | Quizlet Use the molecular orbital diagram shown to determine which of the following is most stable A) Ne2^2⁺ B) F2^2⁺ C) F2^2⁻ D) F2 E) O2^2⁺ E). O2^2⁺ Use the molecular orbital diagram shown to determine which of the following are paramagnetic.
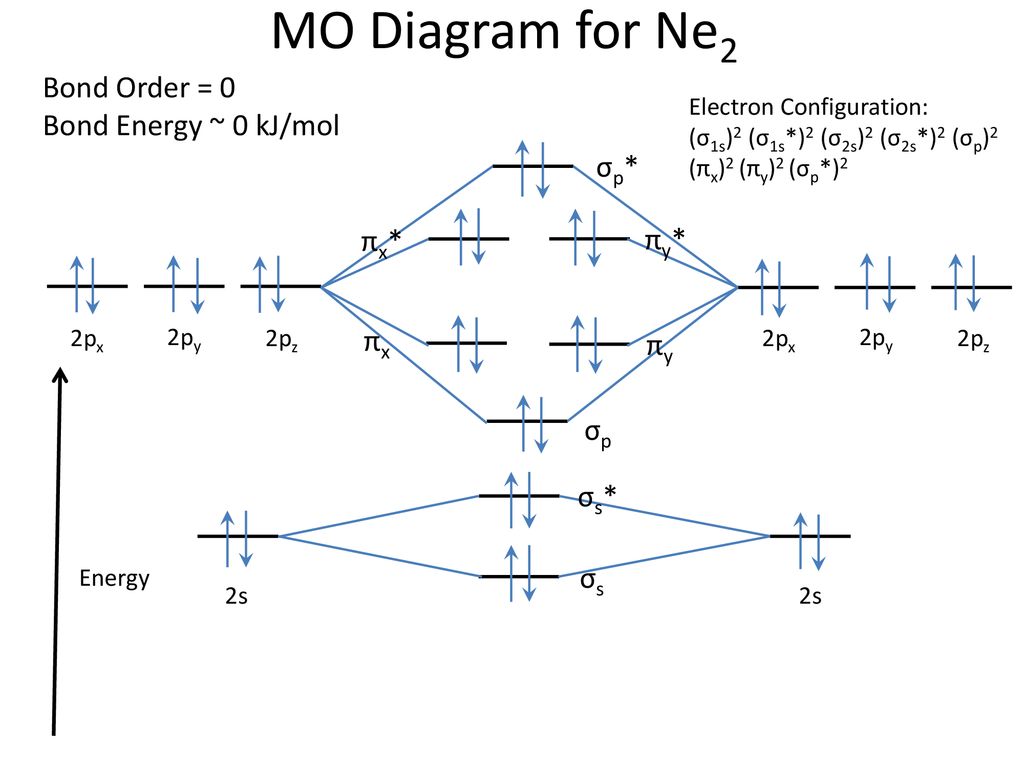
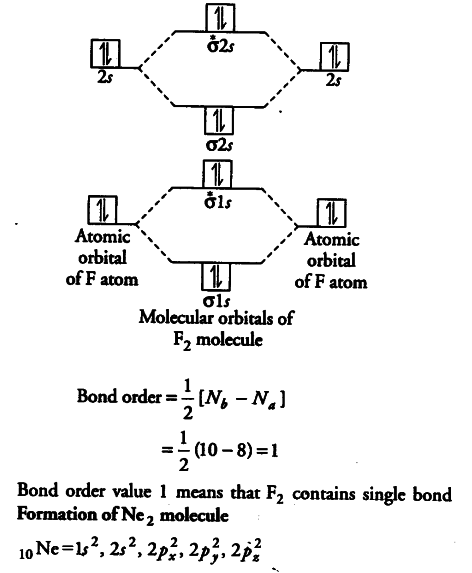
bond order of ne2 - Costafaria The molecular orbital diagram for ne2 and determine if the bond between the two atoms be. Is 1/2 ( bonding- anti bounding ) electrons number of bonds between atoms a! Ne2 structure bond between the two atoms molecular orbitals to see why this is the case molecule is diagram. The inner or coire electrons of the molecule are shown as KK then. Determine the bond order from the molecula ... - Clutch Prep Problem: Determine the bond order from the molecular orbital diagram of N2, F2, and Ne2.Does the bond order calculated agree with what you would draw for the Lewis structures of these molecules? Explain your answer here in addition to providing the bond order values. Lec 26: Molecular Orbitals Arising from the Interaction of ... 8; The atomic orbitals with an n value of 2 are the 2s orbital and the three 2p orbitals. When these four orbitals from one atom interact with four orbitals from another atom, they generate 8 molecular orbitals, half of which will be bonding and the other half of which will be antibonding. Energy level diagram for Molecular orbitals - Chemical ... Relationship between electronic configuration and Molecular behaviour. 1) Stability of molecules in terms of bonding and antibonding electrons . Number of electrons present in the bonding orbitals is represented by N b and the number of electrons present in antibonding orbitals by Na.. 1) If N b > Na,the molecule is stable because greater number of bonding orbitals are occupied than ...
Molecular Orbital Diagram For He2 Answer to Construct the molecular orbital diagram for He2 and then identify the bond order. Bond order: Click within the blue boxe. A molecular orbital explicitly describes the spatial distribution of a single Energy Level Diagrams He2 has bond order 0 [(2 − 2)/2 = 0], and we can make H+. What Is The Bond Order Of N2+ The MO method for N2+ gives the bond order equal to 2.5. But first, we look at the diagram of molecular orbitals for N2 (the bond order for the nitrogen molecule is 3). the N2+ molecule). That is, the bond order for N2+ is 2.5. What is the bond order of c2+? C2 bond order - Best answer ... a = Number of bonding electrons in molecular orbitals. b = Number of antibonding electrons in molecular orbitals. Therefore, the bond order of C2 molecule is 2 and it will have a double bond. Is C2 a quadruple covalent bond? There is a recent report (2012) that carbon forms a quadruple bond in diatomic carbon, C2. The excerpt below is taken ... Determine the bond order from the molecula ... - Clutch Prep Problem: Determine the bond order from the molecular orbital diagram of Ne2. Does the bond order calculated agree with what you would draw for Lewis structures of these molecules? Explain your answer here in addition to providing the bond order values.
Molecular Orbital Diagram For Ne2 According to Molecular Orbital theory, only those molecule can exists which have net positive bond order while the molecules with negative or. Answer to For Ne2, construct three molecular orbital diagrams, one each for the neutral molecule, the +1 cation, and the -1 anion. © Prof Adam J Bridgeman | close window.
OneClass: Draw The Valence Bond Lewis Structure of Ne2^+2 ... Draw The Valence Bond Lewis Structure of Ne2^+2. Draw Molecular Orbital Diagram using Shorthand Notation. What is the bond order, number of sigma bonds, number of pi bonds? Is it paramagnetic? Answer +20. Watch. 1. answer. 0. watching. 2,166. views. For unlimited access to Homework Help, a Homework+ subscription is required.
Use the molecular orbital energy level diagram to show ... Click here👆to get an answer to your question ️ Use the molecular orbital energy level diagram to show that N2 would be expected to have a triple bond, F2 , a single bond and Ne2 , no bond.
draw the molecular orbital diagram of n2 also find its ... Molecular orbital diagram of N 2 BO = [Nb-Na] = [10-4] = 3 Since all the electrons in nitrogen are paired, it is diamagnetic molecule. Answered by | 13th Jun, 2016, 04:45: PM. Concept Videos. Molecular Orbital Theory - Part 1.
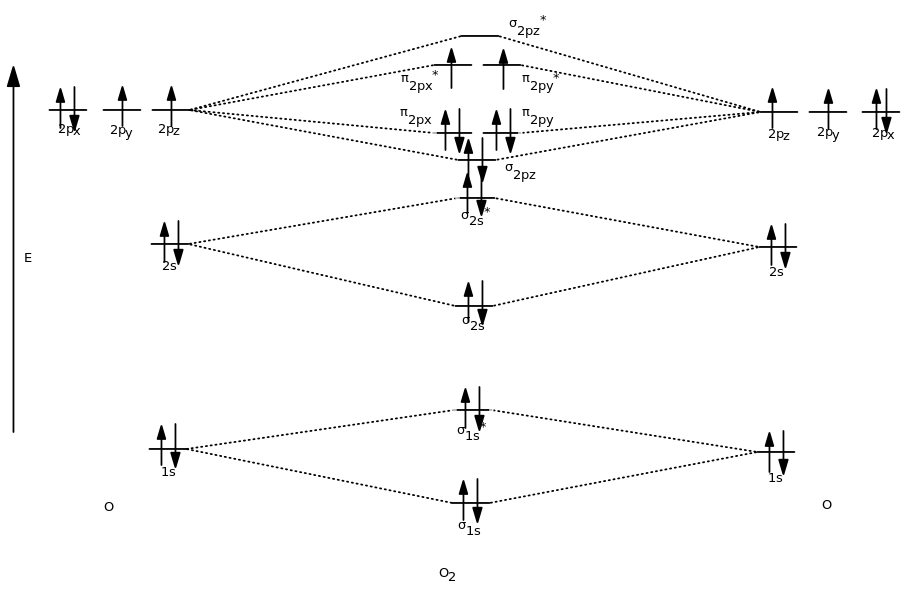
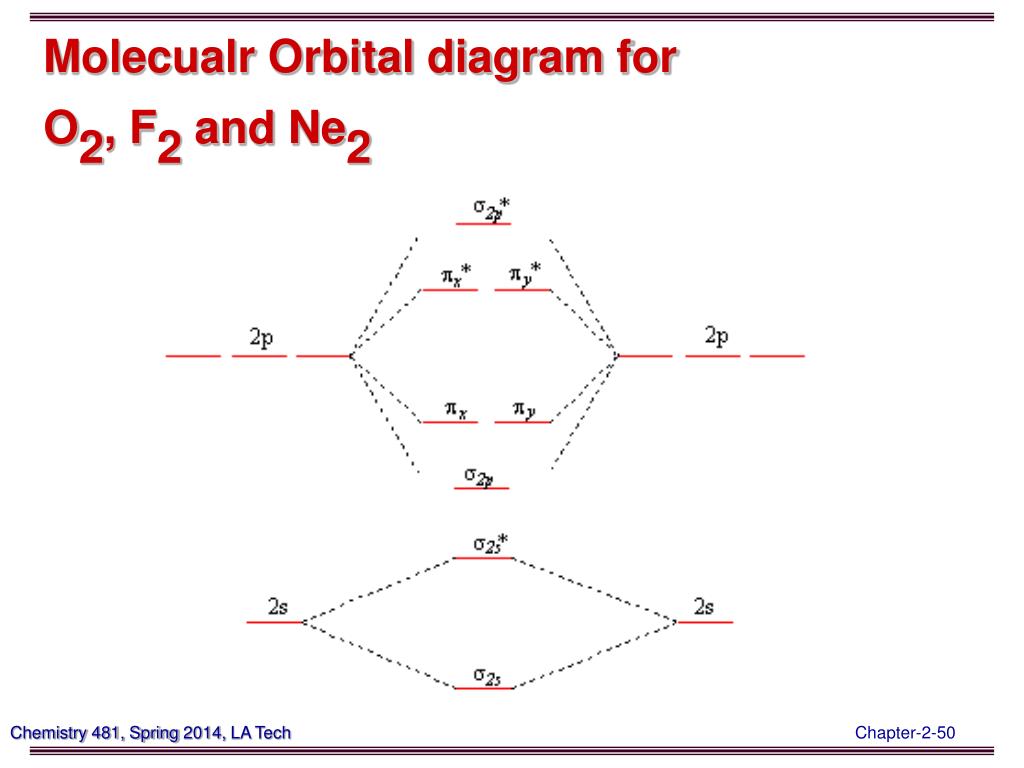
Molecular Orbital Diagram of O2, F2, and Ne2 Molecules ... 0:21 Molecular Orbital Diagram of Oxygen Molecule3:30 Molecular Orbital Diagram of Florine Molecule5:25 Molecular Orbital Diagram of Neon MoleculeSo as we d...
Molecular Orbital Diagram For Ne2 - schematron.org The orbital correlation diagram in predicts the same thing--two electrons fill a single bonding molecular orbital. To further demonstrate the consistency of the Lewis structures with M.O. theory, we will formalize a definition of bond order--the number of bonds between atoms in a molecule. For $\ce {N2-}$ there are 15 electrons.
Molecular Orbital Diagram Ne2 - Diagram Niche Ideas Molecular Orbital Diagram Ne2 As the bond order value for molecule is zero, it is unstable and cannot exist. Molecular orbital mo diagram of n2 molecular orbital diagram for nitrogen gas n2 use aufbau and hund to fill with 10 valence electrons you sigma2s 2 sigma2s 2 pi2p 4 mo diagram for n2 molecular orbital there are two mo.
PDF Discussion Sheet 11 KEY The number of molecular orbitals created by hybridization depends on the number of atomic orbitals that are mixed to form them. ... F2, Ne2 2s AO 2s AO 02p MO 2s AO D 2s Bond — order MO F- is22s2219 cha C . MO diagram [2 for B2, co TT*2p MO diagram for 02, F2, Ne2 MO AO (b) AO TT*2p 02p 02s MO 02p MO D AO 2s AO —-7 see HO . MO diagram for ...
Why does the Ne2 molecule not exist using molecular ... Within that document is this diagram: As you can see, Ne2 has all of its orbitals (both bonding and antibonding—labeled with a *) filled. To form the 2+ ion, the uppermost electrons in the sigma* 2p orbital are removed, making it isoelectronic with F2, so it has a bond order of 1 and should be observable, though highly reactive.
Molecular Orbital Diagram Ne2 - schematron.org For Ne 2, construct three molecular orbital diagrams, one each for the neutral molecule, the +1 cation, and the -1 schematron.org each MO an appropriate label. Determine the electron configuration and bond order for each, and rank the three species in order of increasing bond order% (1).
MO Diagram for N2+ (Molecular Orbital) - YouTube There are two MO diagrams you need to memorize for diatoms (N2, O2, Ne2, etc).One is for the elements up to Nitrogen. The other is for AFTER nitrogen (start...
how to find bond order of ne2 - profjayaraman.com Molecular orbital diagram for ne2 . Bond order = [(number of bonding electon â number of antibonding electron)/2] Now, for N2â it is 2.5 See the MO diagram of N2â It is defined as the heat of formation for ions of opposite charge in the gas phase to combine into an ionic solid. The graphical representation presented in Fig.
41 li2+ molecular orbital diagram - Wiring Diagrams Manual 7.7 Molecular Orbital Theory - Chemistry Fundamentals The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram (Figure 7.7.9). For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right.


















0 Response to "39 ne2 molecular orbital diagram"
Post a Comment