41 bromine lewis dot diagram
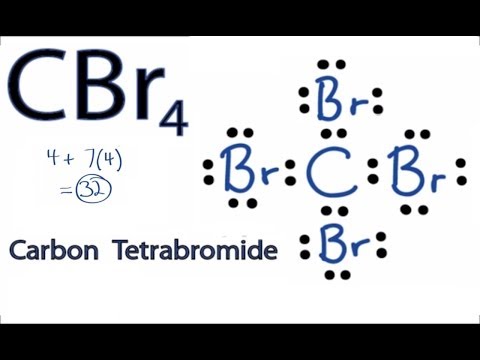
CBr4 Lewis Structure - How to Draw the Dot Structure for ... Transcript: This is the CBr4 Lewis structure: Carbon Tetrabromide. Carbon is in group 4 or 14, so it has 4 valence electrons. Bromine in group 7 or 17, so it has 7, and we have 4 Bromines. So 4 plus 28 equals 32 total valence electrons. Carbon, that's the least electronegative, that'll go in the center; and on the outside we'll put the Bromine ... How to draw Br2 Lewis Structure - Science Education and ... Step-1: Br2 Lewis dot Structure by counting valence electrons on the bromine atom. To calculate the valence electron of each atom in Br2, look for its periodic group from the periodic table. The halogen group families, which is the 17th in the periodic table, are made up of two bromine atoms.
Lewis Dot Diagram - Organic Chemistry - Socratic The Lewis dot diagram for the covalent bonding of chlorine, ( Cl2 ), would be: When atoms are bonded ionically, the bond is represented by two dots between the element's chemical symbols. Ionic bonds are formed between charged particles (ions), so an example of an ionic compound would be NaCl, whose Lewis structure is: YouTube. chemistNATE.

Bromine lewis dot diagram
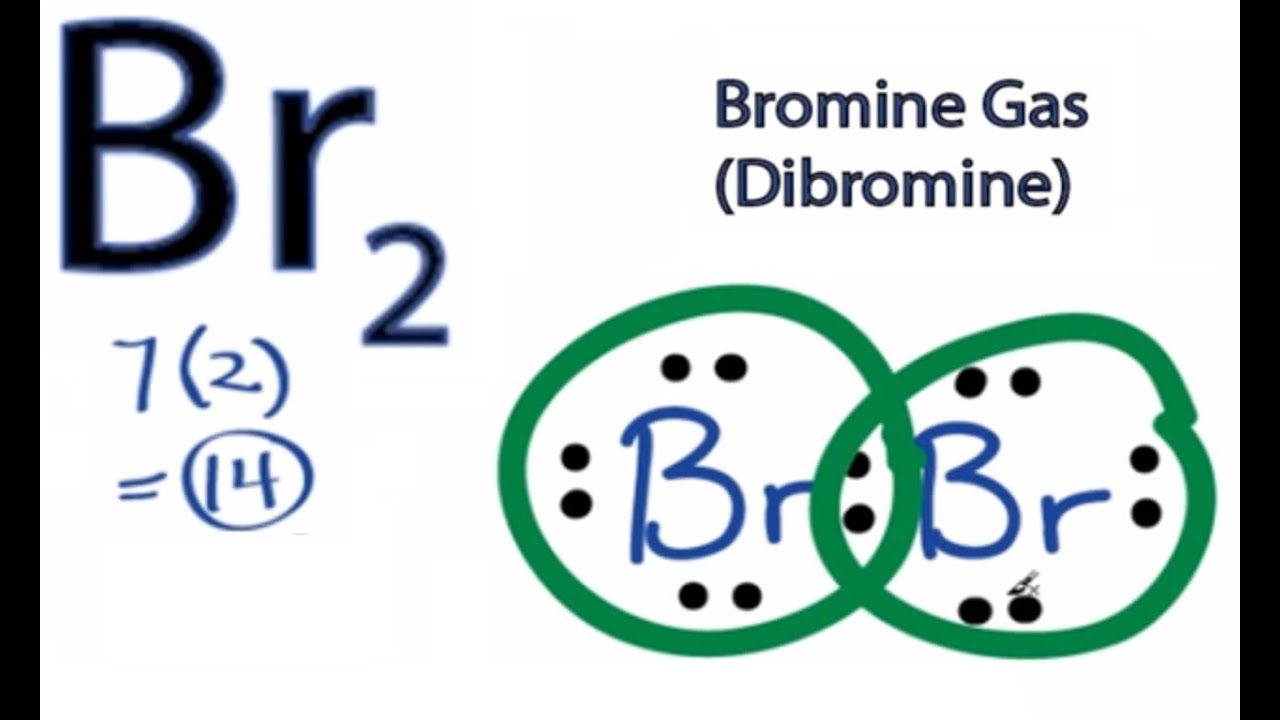
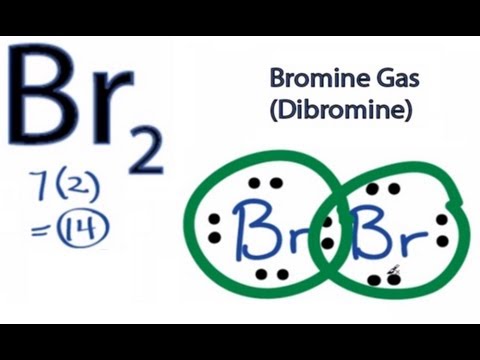
Lewis Dot Diagrams (Structures) for Atoms and Ions ... Hydrogen / Helium (watch out!) / Bromine Selenium / Nitrogen / Barium Chlorine / Gallium / Argon. WKS 6.2 - LDS for Ions/ Typical Charges. Determine the common oxidation number (charge) for each of the following ions, and then draw their Lewis Dot Structure. Don't forget to show brackets and charge on your LDS for ions! Chemical Bonding: Br 2 Lewis Structure - The Geoexchange Br2 Lewis Structure - How to Draw the Lewis Dot Structure for Dibromine Watch later Watch on Transcript: This is the Br2 Lewis structure. Looking on the periodic table, Bromine is in Group 7 or 17. It has 7 valence electrons. We have two of them though. Multiply that by 2, for a total of 14 valence electrons for the Br2 Lewis structure. Brf3 Lewis Structure: Draw the Bromine Trifluoride Dot ... BrF3 Lewis Structure (Bromine Trifluoride) Watch later Watch on A Lewis dot structure or electron dot structure is a diagram that shows the bondings of the atoms in the molecule along with their lone pairs. The bonds in the diagram are shown by using lines, whereas the lone pairs are represented as dots.
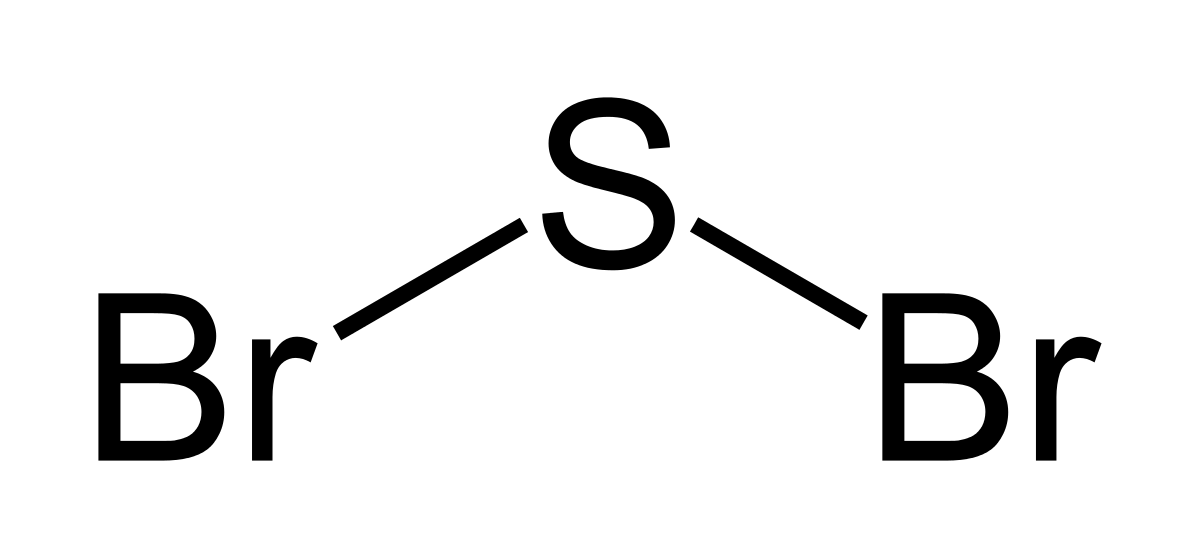
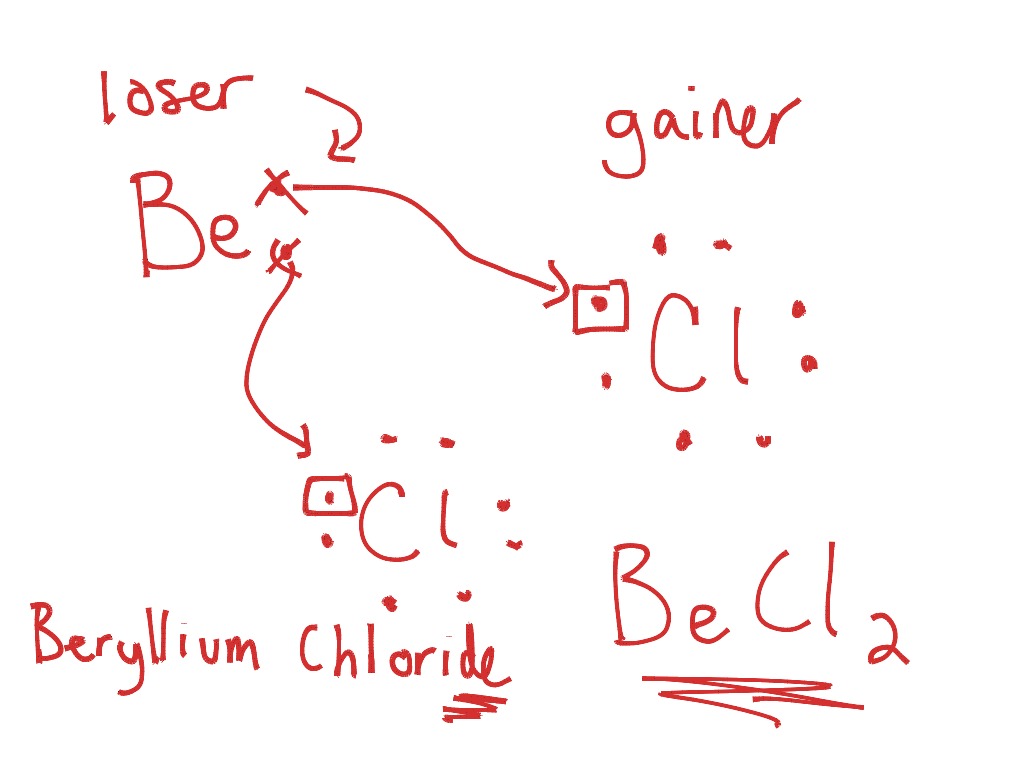
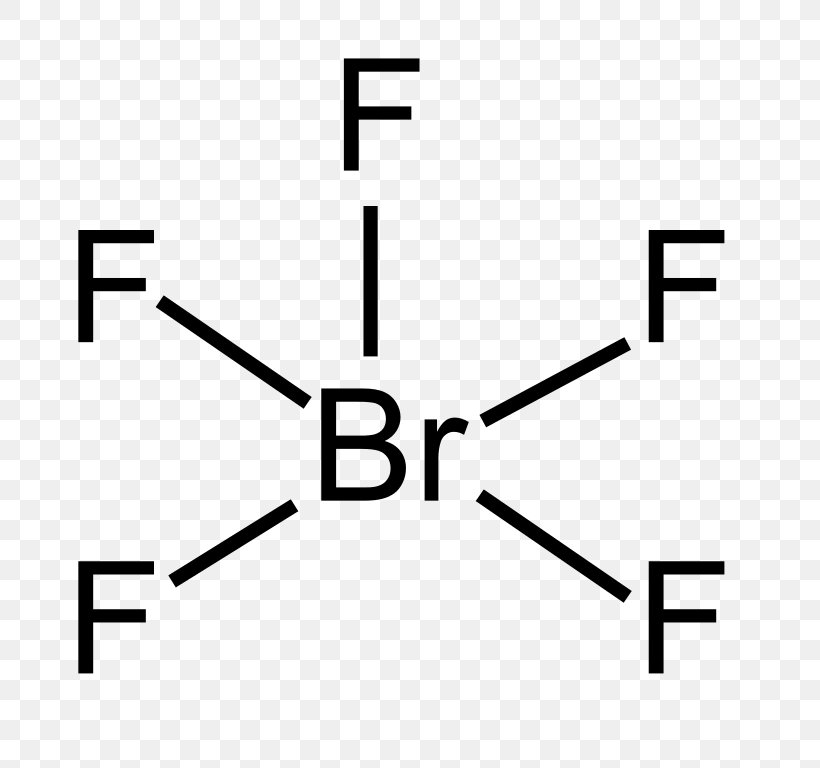
Bromine lewis dot diagram. How to Draw the Lewis Dot Structure for Br ( the Element ... A step-by-step explanation of how to draw the Br Lewis Dot Structure.For the Br structure use the periodic table to find the total number of valence electron... How to draw BeBr2 Lewis Structure? - Science Education and ... It is represented by dots in the BeBr2 Lewis diagram. The BeBr2 molecule's core carbon atom can be represented as follows: Total outermost valence shell electron of beryllium atom in BeBr2= 2 Total outermost valence shell electron of atom in BeBr2= 7 The BeBr2 molecule has one central beryllium atom and two bromine atoms. How to draw BrF3 Lewis Structure? - Science Education and ... Key Points To Consider When Drawing The BrF3 Electron Dot Structure. A three-step approach for drawing the BrF3 Lewis structure can be used. The first step is to sketch the Lewis structure of the BrF3 molecule, to add valence electrons around the bromine atom; the second step is to add valence electrons to the three fluorine atoms, and the final step is to combine the step1 and step2 to get ... 6.1 Lewis Electron Dot Diagrams | Introductory Chemistry A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom.
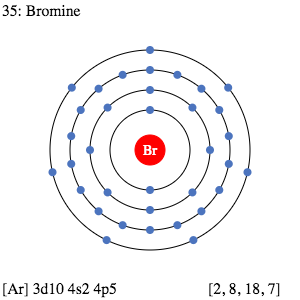
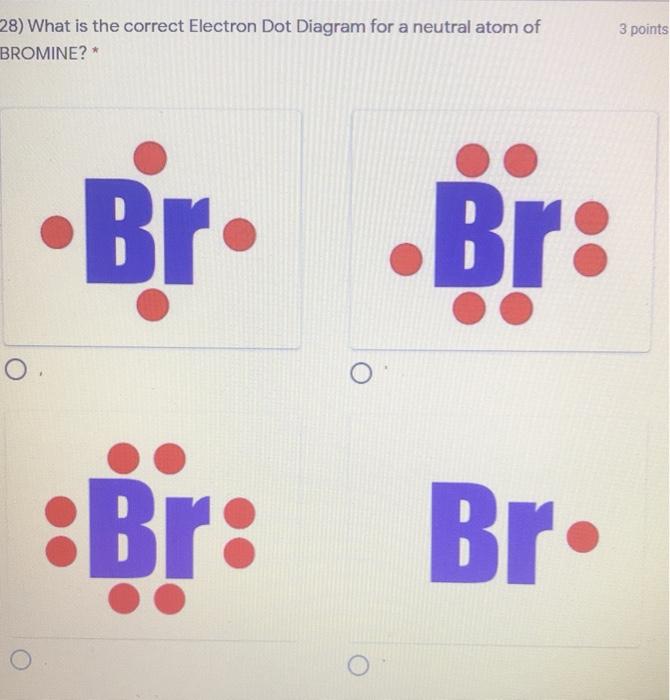
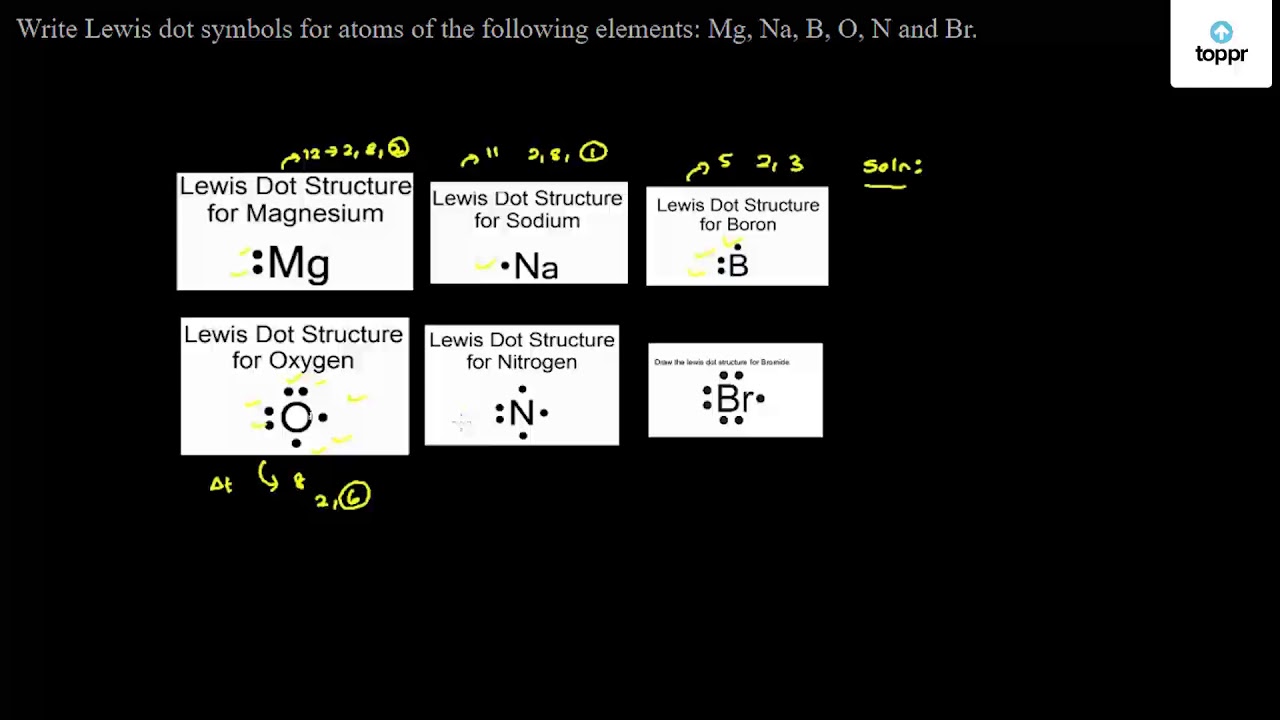
Bromine - Home Bromine is a nonmetal. Bromine Electron Dot Diagram Since Bromine is in group, or series, 17, it has 7 valence electrons. Group 1 on the periodic table has 1 valence electron. Group 2 on the periodic table has 2 valence electrons. The amount of valence electrons in groups 3-12 cannot be predicted because their oxidation numbers are unknown. Write out the electron configuration for neutral bromine. Draw ... 17 Feb 2018 — What is the lewis structure for hcn? How is vsepr used to classify molecules? What are the units used for the ideal gas law? How does Charle's ...1 answer · Well the bromine atom has 7 valence electrons.... Explanation: And there is a single bromine-bromine bond in the Br2 molecule... bluemindmonkeys.com ... How to Draw the Lewis Dot Structure for BrI5: Bromine ... A step-by-step explanation of how to draw the BrI5 Lewis Dot Structure (Bromine pentaiodide).For the BrI5 structure use the periodic table to find the total ... Lewis dot structure for Bromine and Iodine? - Answers Bromine (Br) has seven valence electrons. This means that the Lewis dot structure has two dots above, below and to the left, and one dot to the right.
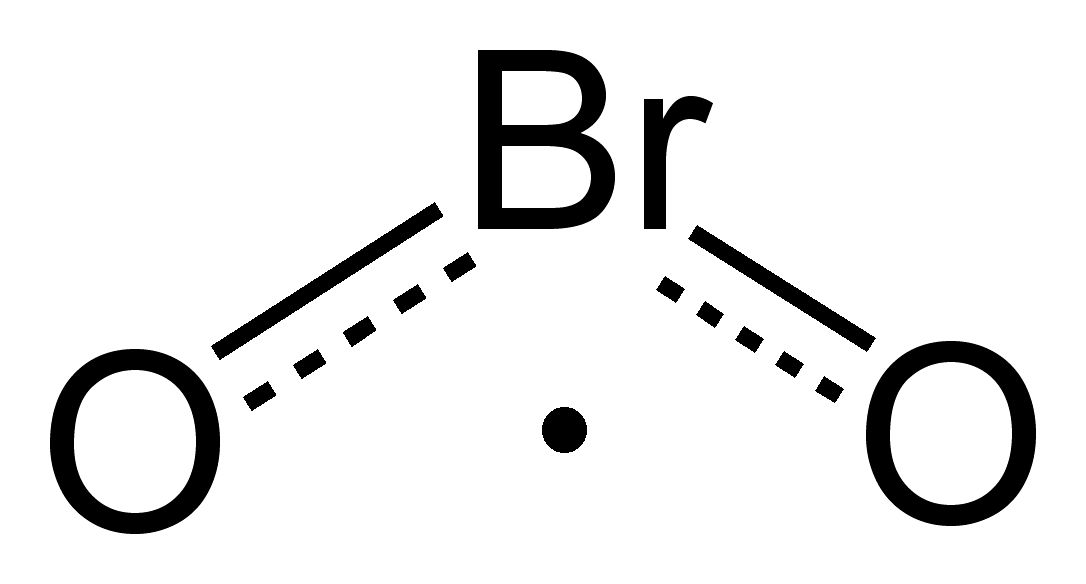
How to Draw the Lewis Dot Structure for BrI3: Bromine ... A step-by-step explanation of how to draw the BrI3 Lewis Dot Structure.For the BrI3 structure use the periodic table to find the total number of valence elec... How to Draw the Lewis Dot Structure for NaBr: Sodium ... A step-by-step explanation of how to draw the NaBr Lewis Dot Structure.For NaBr we have an ionic compound and we need to take that into account when we draw ... Bromine pentafluoride (BrF5) lewis dot structure ... BrF5 lewis dot structure has 1 bromine and 5 fluorine atom. There is one lone pair present on bromine and it is connected with 5 fluorine atoms with the help of five single bonds. Follow some steps for drawing the lewis dot structure of BrF5. 1. Count total valence electron in BrF5. PDF Lewis Dot Structures - University of Pennsylvania School ... 1. a) Draw the Lewis dot structure for an atom of carbon and an atom of bromine. b) Using the NEED, HAVE, SHARE method, determine the number of valence electrons that would be shared between one carbon atom and four bromine atoms. c) Draw the Lewis dot structure for the compound CBr 4. 2. a) Draw the Lewis dot structure for an atom of iodine.
Br2 Lewis Structure - How to Draw the Lewis Dot Structure ... A step-by-step explanation of how to draw the Br2 Lewis Dot Structure (Bromine gas).For the Br2 structure use the periodic table to find the total number of ...
How to Draw the Lewis Dot Structure for BrCl: Bromine ... A step-by-step explanation of how to draw the BrCl Lewis Dot Structure (Bromine monochloride).For the BrCl structure use the periodic table to find the total...
Bromine (Br2) Lewis Structure - chemistryscl.com Bromine (Br 2) Molecule Lewis Structure Bromine is a diatomic molecule and contains only bromine atoms. Lewis structure of bromine contains only one Br-Br bond and each bromine atom has three lone pairs. It is very easy to Br 2 lewis structure. Br 2 lewis structure
Bromine Bohr Model - How to draw Bohr diagram for Bromine ... Electron dot diagram of a Bromine atom. Electron dot diagram also called lewis structure which represents the valence electrons of atoms. As, from the Bohr diagram of Bromine, we got to know, it has 7 valence electrons. So, just represent the 7 valence electron around the Bromine atom as a dot.
Draw the Lewis dot diagram for bromine. | Study.com The Lewis structure generally provides knowledge about the representation of valence electrons through the solid dot around the nucleus of the given element. It ...1 answer · Top answer: As we all know the atomic number of bromine is 35 and it is one of the halogen gas. The electronic configuration of bromine is...
Brf3 Lewis Structure: Draw the Bromine Trifluoride Dot ... BrF3 Lewis Structure (Bromine Trifluoride) Watch later Watch on A Lewis dot structure or electron dot structure is a diagram that shows the bondings of the atoms in the molecule along with their lone pairs. The bonds in the diagram are shown by using lines, whereas the lone pairs are represented as dots.
Chemical Bonding: Br 2 Lewis Structure - The Geoexchange Br2 Lewis Structure - How to Draw the Lewis Dot Structure for Dibromine Watch later Watch on Transcript: This is the Br2 Lewis structure. Looking on the periodic table, Bromine is in Group 7 or 17. It has 7 valence electrons. We have two of them though. Multiply that by 2, for a total of 14 valence electrons for the Br2 Lewis structure.
Lewis Dot Diagrams (Structures) for Atoms and Ions ... Hydrogen / Helium (watch out!) / Bromine Selenium / Nitrogen / Barium Chlorine / Gallium / Argon. WKS 6.2 - LDS for Ions/ Typical Charges. Determine the common oxidation number (charge) for each of the following ions, and then draw their Lewis Dot Structure. Don't forget to show brackets and charge on your LDS for ions!



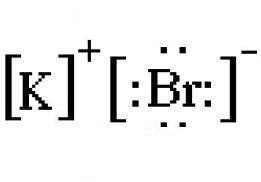





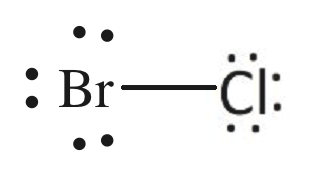












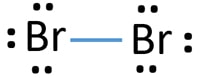
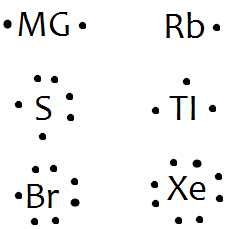



0 Response to "41 bromine lewis dot diagram"
Post a Comment