42 match the appropriate octahedral crystal-field splitting diagram fe2+
Crystal field theory - Wikipedia Crystal field theory (CFT) describes the breaking of degeneracies of electron orbital states, usually d or f orbitals, due to a static electric field produced by a surrounding charge distribution (anion neighbors). Match The Appropriate Octahedral Crystal Field Splitting Diagram... Crystal field splitting in an octahedral field eg energy 35 o o 25 o t2g e g the higher energy set of orbitals d z2 and d x2 y2 t 2g the lower energy set of Lecture 7 crystal field theory for octahedral complexes. High spin cr2 low spin fe3 please give the octahedral splitting diagrams for each ion.
Crystal field theory: Splitting of orbitals in Octahedral complexes Hi students! This video is about Crystal field theory (CFT) of coordination compounds.Due to technical error some words are missing from the video, please...

Match the appropriate octahedral crystal-field splitting diagram fe2+
Draw figure to show the splitting of d orbitals in an octahedral crystal... > The crystal field splitting for Cr3+ ion in octahedral field changes for following ligands in decreasing order as > The magnitude of CFSE (Crystal Field Splitting Energy, Δ0 ) can be related to the configuration of d - orbitals in a coordination entity as? Answered: Match the appropriate octahedral… | bartleby Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion and spin state Octahedral splitting diagram Answer Bank high-spin Mn2+ 1 11 1L 1 1 11 11 low-spin Ni3+ 11 111 1L 1.1 1L 11 11 11 11 1 |1 11 111. How do p-orbitals and d-orbitals split in an octahedral crystal field? Crystal field splitting for octahedral complex is: So, because of above reason in dx2-y2 and dz2 there is repulsion between the orbitals and ligands and it goes higher in You can calculate crystal field splitting energy by using above diagram for various complexes with six number of ligands present.
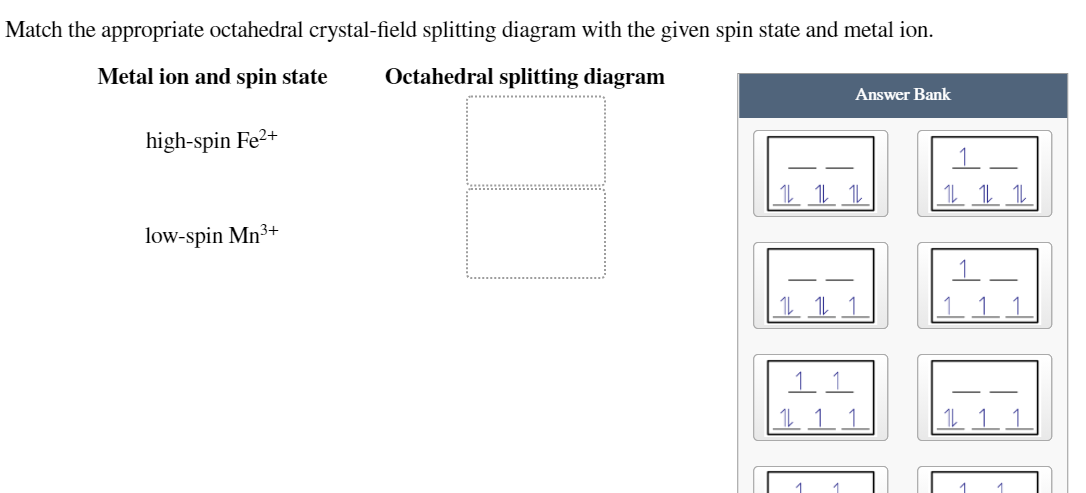
Match the appropriate octahedral crystal-field splitting diagram fe2+. (PDF) Chemistry - Gilbert | Tín Phạm - Academia.edu Academia.edu is a platform for academics to share research papers. 34 Match The Appropriate Octahedral Crystal Field Splitting... Crystal field theory octahedral geometry. High spin cr2 low spin fe3 please give the octahedral splitting diagrams for each ion. Hw Solutions 5 Chemistry Libretexts. Oxidation State And Coordination Environment Of Pb In U Bearing. Solved Match The Appropriate Octahedral Crystal... Solved Match The Appropriate Octahedral Crystal | Best Diagram... Leave a Reply Cancel reply. Your email address will not be published. Required fields are marked *. Crystal Field Theory | Octahedral Crystal Fields Octahedral Crystal Fields. Each Mn2+ ion in manganese(II) oxide is surrounded by six O2- ions arranged toward the corners of an octahedron, as shown in the figure below. MnO is therefore a model for an octahedral complex in which a transition-metal ion is coordinated to six ligands.
Crystal field theory Square planar crystal field Crystal field splitting parameters CFSE. 2. Page 2 of 33 Introduction The bonding of transition metal complexes can be 3. Page 3 of 33 The Octahedral Crystal Field Consider an octahedral arrangement of ligands around the metal. The lone pair of electrons on each... SOLVED:Match the appropriate octahedral crystal-field splitting... Doctor Octopus Federal crystal splitting field. Chromium three. Plus Looks like that for copper. Answer. Draw the octahedral crystal field splitting diagram for each metal ion. a. Cr3+ c. Mn3+ b. Cu2+(high- and low-spin) d. Fe2+( low-spin). Match The Appropriate Octahedral Crystal Field Splitting Diagram appropriate octahedral crystal-field splitting diagram mn2+ › match the appropriate octahedral crystal-field splitting diagram mn3+ Given this is an octahedral complex your splitting diagram will have 2 degenerate states. Introduction To Inorganic Chemistry Coordination Chemistry And. Crystal Field Theory - Chemistry LibreTexts | Octahedral Complexes Crystal field theory (CFT) describes the breaking of orbital degeneracy in transition metal Ligands that produce a large crystal field splitting, which leads to low spin, ar e called strong field ligands . A tetrahedral complex absorbs at 545 nm. What is the respective octahedral crystal field splitting...
CBSE Free NCERT Solution of 12th chemistry... | SaralStudy Q18 What is crystal field splitting energy? How does the magnitude of Δo decide the actual configuration of Q20 A solution of [Ni(H2O)6]2+ is green but a solution of [Ni(CN)4]2- is colourless. The splitting of the d orbitals in an octahedral field takes palce in such a way that dx2y2 , dz2... PDF Outline of crystal field theory | 3.4.2 Tanabe-Sugano Diagrams effect of crystal field splitting is to cause electrons to populate 3d orbitals having. the lowest energy. expected to show strong preferences for octahedral coordination sites. Cations with 3tfJ, 3d10 and high-spin 3tF configurations, such as Ca2+, Zn2+, Mn2+ and Fe3+, receive zero CFSE in... Octahedral crystal field splitting parameter - Big Chemical... Second, the octahedral crystal field splitting parameters, values of which are higher for smaller sites, are expected to decrease in the same order as eq. The use of the crystal field splitting parameter would seem to be a more sensible parameter to use than the position of Amax for the main absorption... 27 Match The Appropriate Octahedral Crystal Field Splitting... As a result the splitting observed in a tetrahedral crystal field is the opposite of the splitting in an octahedral complex. Match the appropriate octahedral crystal field splitting diagram with the given spin state and metal ion. High spin cr2 low spin fe3 please give the octahedral splitting...
D orbital splitting diagram? Fe(CN) 6 4- - HomeworkLib What is the octahedral crystal-field splitting diagram for the metal Fe(CN)6 4 Match the appropriate octahedral crystal-field splitting diagram with the given spin... Metal ion and spin state Octahedral splitting diagram Answer Bank high-spin Co2+ 1 1 1 1 1 low-spin Mn2+ | 11 1 1 1...
Chapter 22: Metal Complexes - ppt video online download 55 Octahedral Crystal Field Splitting (dx2 - y2, dz2) (dxy, dxz, dyz) = Crystal field field ligand. 61 Your Turn! Which of the following complexes will have the largest Δ splitting? 69 Using Crystal Field Theory Cr2+ has d 4 electron configuration First three go into lower level according to Hund's...
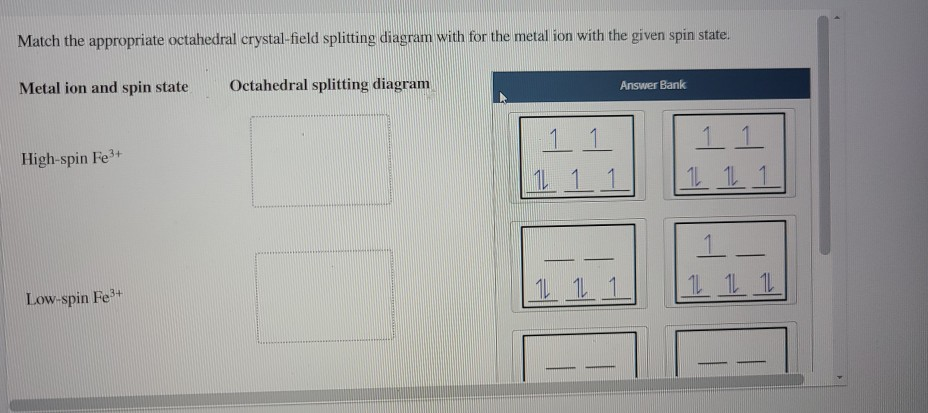
[Solved] 16 of 25 Att Match the appropriate octahedral crystal-field... Metal ion and spin state Octahedral splitting diagram Answer Bank High-spin Fe3+ 1 1 14 14 1 Low-spin Fe3+ 1 1 1 1 1 1 -1 1 11 12 11 1 1 11 1 1 14 1 1. Q: Please help me with this question, thanks. Construct the octahedral crystal-field splitting diagram for the metal in eac.
PDF Microsoft PowerPoint - Chapter 20 - d-block metal chemistry... Octahedral crystal field stabilization energies (CFSE) for dn configurations; pairing energy, P, terms are included where appropriate. Determine the values of Δo and B using a Tanabe Sugano Diagram (at right or handout) 1. Assign the transitions of the two most intense.
FIG. 5. (Color online) (a) Octahedral crystal field splitting. (b)... Download scientific diagram | (Color online) (a) Octahedral crystal field splitting. (b) Linear Splitting is exaggerated out of proportion to the exact ratio with octahedral field splitting for The crystal-field splitting energy increases with increasing pressure, which can favor the low-spin state.
Draw the figure to show the splitting of 'd' orbitals in octahedral... Answer: d-orbitals in octahedral complex split into two energy levels:The lower energy Please refer to the diagram of splitting of a d-orbitals in the attached image.... hendikeps2 and 5 more users Explanation:The splitting of the d orbitals in an octahedral field takes palce in such a way that dx2y2...
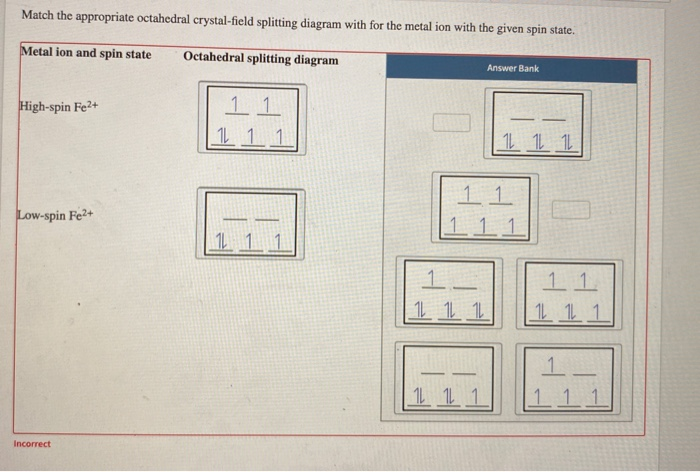
Solved Match the appropriate octahedral crystal-field | Chegg.com Chemistry questions and answers. Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. High spin Cr2+ Low spin Fe 2+.
Crystal Field Theory Crystal field splitting does not change the total energy of the d orbitals. Table 23.10 Crystal Field Splitting Energies for Some Octahedral (Δo)* and Tetrahedral (Δt) Transition-Metal Complexes. Exactly the same effects are seen in the second half of the first-row transition metals. In the Fe2+...
Construct the octahedral crystal-field splitting diagram for the metal... Calculate the crystal field splitting energy (in kJ/mol) for this ion. Difracction problem. Diffraction from an unknown cubic metal is observed to occur at An octahedral die has eight faces numbered 1 to 8, each of which comes up with equal likelihood (you can see a picture of one in Diagram 3 of the Test 1...
Crystal Field Theory (CFT). To predict the splitting pattern of the energy of the d-orbitals under a tetrahedal crystal field you may once again That is, the exact opposite of the situation we just dealt with for the octahedral crystal field. Note that a different CFT energy splitting diagram has to be applied for each stereochemistry.
Crystal Field Splitting - an overview | ScienceDirect Topics Crystal field splitting, chain packing and solvation interact with the molecular unit by coupling external electric fields with the electronic structure of the Crystal field d orbital splitting diagrams for common geometries. The above treatment considers the ligands in an octahedral geometry (i.e., with...
Match The Appropriate Octahedral Crystal Field Splitting Diagram... Home › match the appropriate octahedral crystal-field splitting diagram fe2+. Mno4 h fe2 mn2 h2o fe3 asked by claire on june 29 2009. Cobalt 3 has 6 d electrons cobalt normally has 9 valence electrons but youve lost 3. As a result the splitting observed in a tetrahedral crystal field is the...
draw the octahedral crystal field splitting diagram for each metal ion a cr3 b cu2 c mn3 high and lo
Match the appropriate octahedral crystal-field splitting diagram... Get the detailed answer: Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion and spin state Octahedral Splitting Diagram High Spin Fe^3+ Low Spin Co^2+.
How do p-orbitals and d-orbitals split in an octahedral crystal field? Crystal field splitting for octahedral complex is: So, because of above reason in dx2-y2 and dz2 there is repulsion between the orbitals and ligands and it goes higher in You can calculate crystal field splitting energy by using above diagram for various complexes with six number of ligands present.
Answered: Match the appropriate octahedral… | bartleby Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion and spin state Octahedral splitting diagram Answer Bank high-spin Mn2+ 1 11 1L 1 1 11 11 low-spin Ni3+ 11 111 1L 1.1 1L 11 11 11 11 1 |1 11 111.
Draw figure to show the splitting of d orbitals in an octahedral crystal... > The crystal field splitting for Cr3+ ion in octahedral field changes for following ligands in decreasing order as > The magnitude of CFSE (Crystal Field Splitting Energy, Δ0 ) can be related to the configuration of d - orbitals in a coordination entity as?
![Frontiers | New Cationic fac-[Re(CO)3(deeb)B2]+ Complex ...](https://www.frontiersin.org/files/Articles/647816/fchem-09-647816-HTML-r1/image_m/fchem-09-647816-g003.jpg)




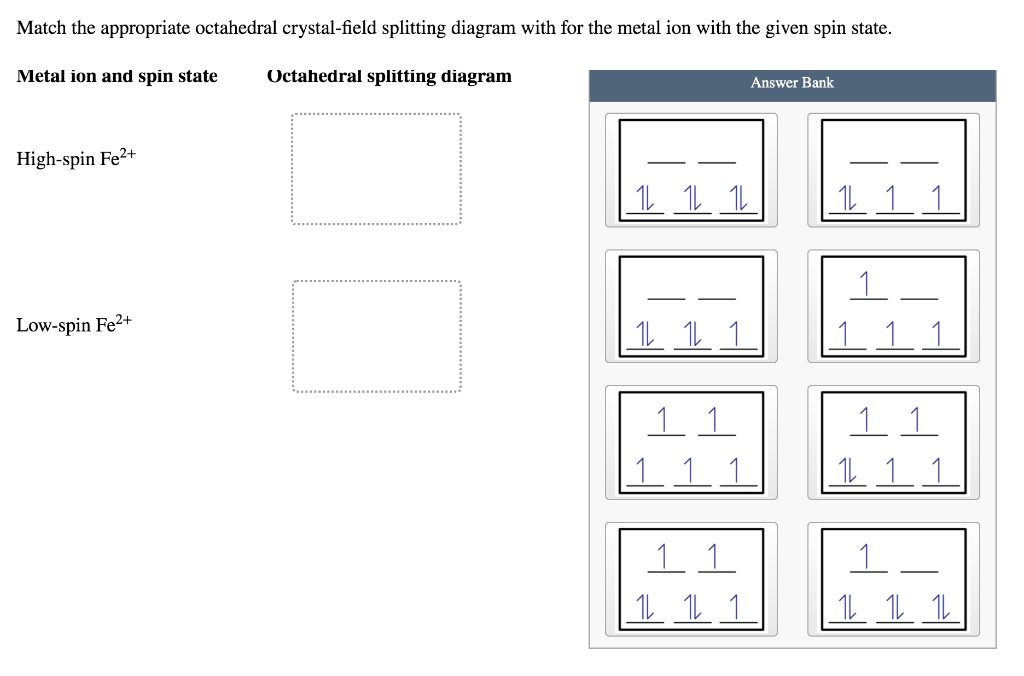
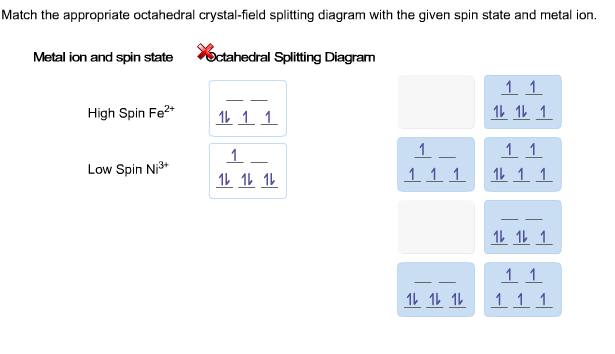











0 Response to "42 match the appropriate octahedral crystal-field splitting diagram fe2+"
Post a Comment