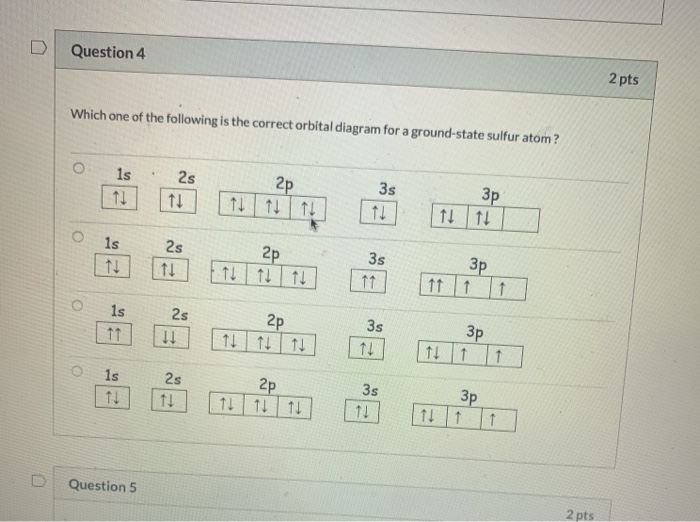
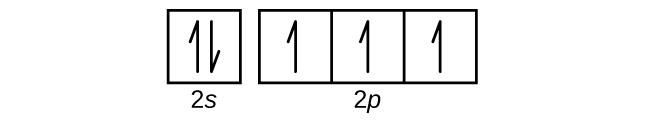
42 orbital filling diagram for sulfur
C2H2 Lewis Structure, Molecular Geometry, Hybridization ... Il y a 1 jour · Filling the number of valence electrons in outermost shells depends on the maximum capacity of a shell and its flexibility in exceptional conditions. As Hydrogen can withhold a maximum of two electrons and carbon can eight, so is the case. It might be interesting for you to realize that there are certain elements, like sulfur, which do not obey the octet rule and can … Electron Configuration Questions and Answers - Study.com Draw the orbital electron filling diagram, using the shortcut of (noble gas) to represent core electrons and up/down arrows to indicate all other electrons, for the …
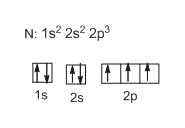
Electron Configurations PowerPoint orbital, or one box from the previous diagram. • Each room on each floor can hold up to 2 people (the electrons), and each room is filled with one person first before the rooms become double occupancy. • Only a man and a woman can live together in a room in the apartment house. (this represents the “spin” of the electrons – one has an up-spin, the other has a down-spin) Who …

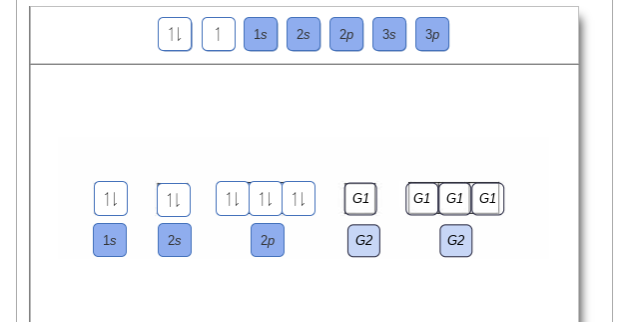
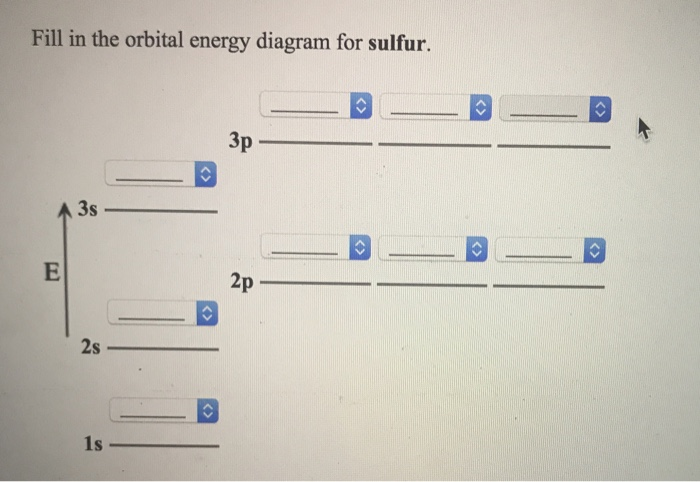
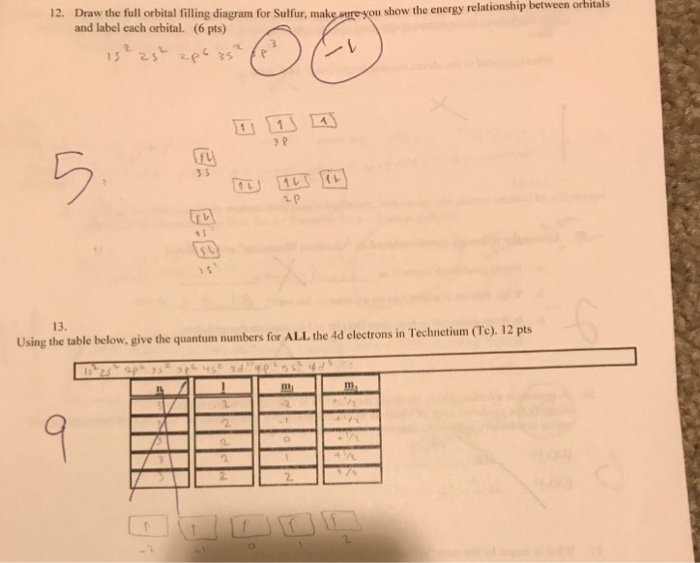
Orbital filling diagram for sulfur
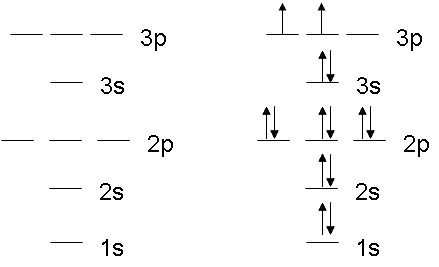
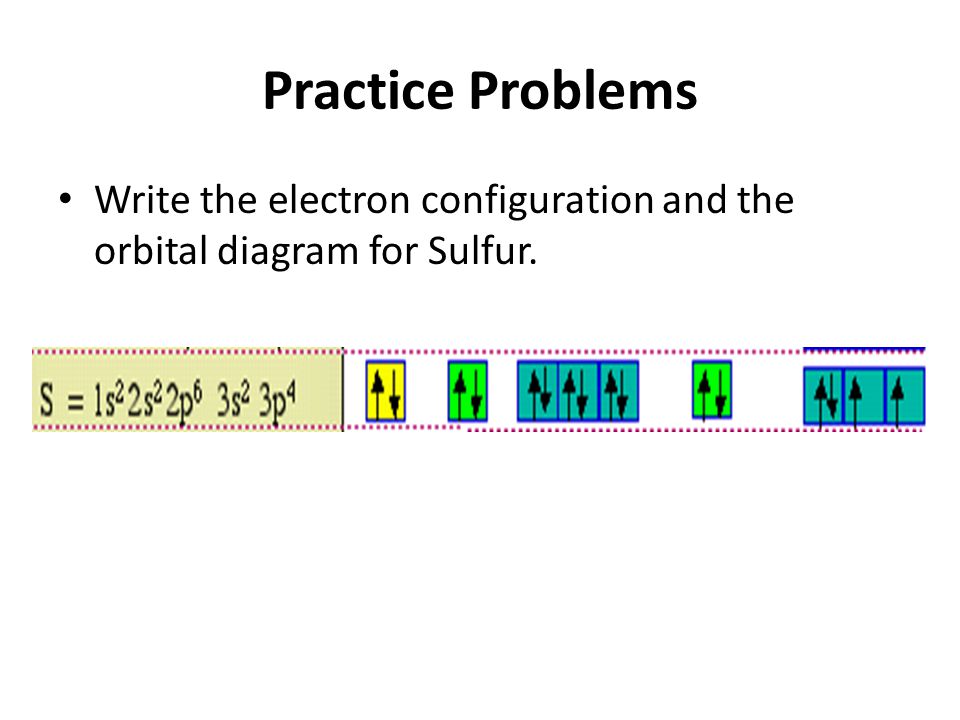
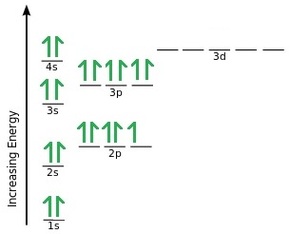
Electron Configurations - Department of Chemistry ... Orbital Diagrams. Another way to represent the order of fill for an atom is by using an orbital diagram often referred to as "the little boxes": The boxes are used to represent the orbitals and to show the electrons placed in them. The order of fill is the same but as you can see from above the electrons are placed singly into the boxes before ... The Electron Configurations of Atoms - Chemistry at Illinois The fifth electron is added to a 2p orbital, the sublevel next higher in energy (Figure 5.9). The electron configuration of boron is: B: 1s 2 2s 2 2p 1. Table 5.2 shows the electron configurations of the elements with atomic numbers 1 through 18. The electron configurations of elements with higher atomic number can be written by following the orbital-filling chart in Figure 5.9. TABLE … Electron Configuration for Sulfur (S) - TerpConnect In writing the electron configuration for Sulfur the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons ...Oct 24, 2016 · Uploaded by Wayne Breslyn
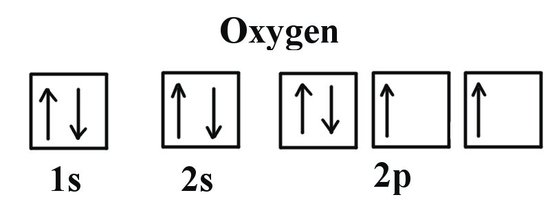
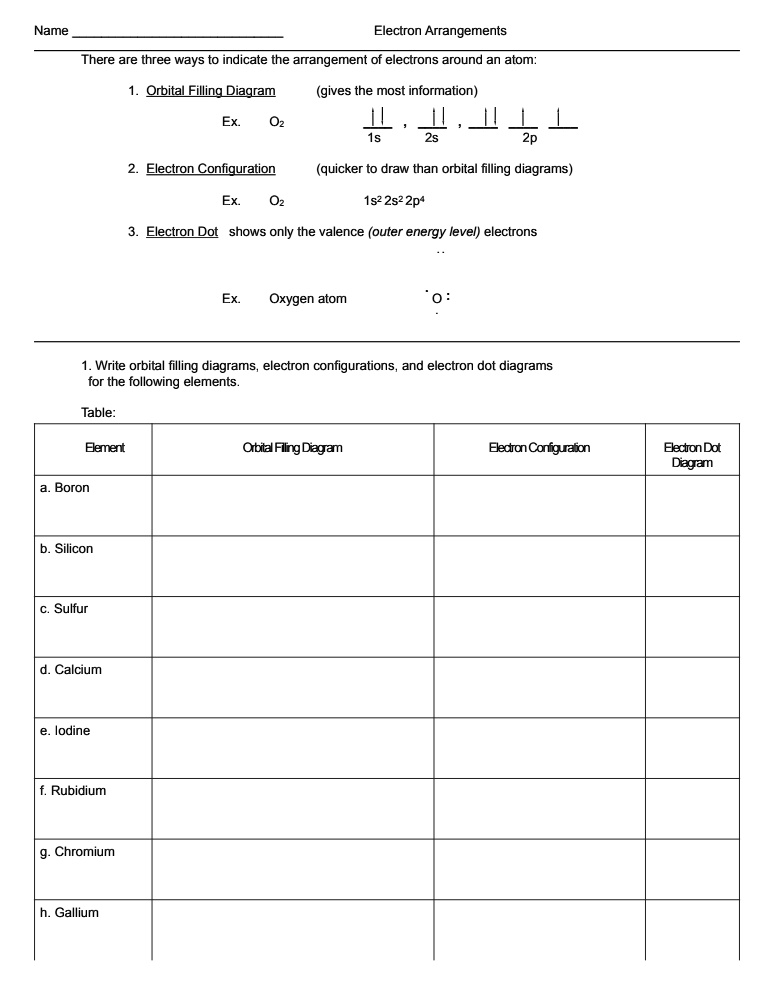
Orbital filling diagram for sulfur. Show the orbital-filling diagram for sulfur. | Study.com These electrons are arranged in increasing order of energy. While filling this orbital, one should take care that first inner shells with less energy are filled ...1 answer · Top answer: Sulfur is an element that belongs to the p-block. The atomic number of sulfur is 16, so contains 16 electrons arranged in increasing order of energy... Electron configuration - Wikipedia Electron configuration was first conceived under the Bohr model of the atom, and it is still common to speak of shells and subshells despite the advances in understanding of the quantum-mechanical nature of electrons.. An electron shell is the set of allowed states that share the same principal quantum number, n (the number before the letter in the orbital label), that electrons … Electron Configuration Practice Worksheet - Weebly 1. Write orbital filling diagrams, electron configurations, and electron dot diagrams for the following elements. Table: Element Orbital Filling Diagram Electron Configuration Electron Dot Diagram a. Boron b. Silicon c. Sulfur d. Calcium e. Iodine f. Rubidium g. Chromium h. Gallium 8.4 Molecular Orbital Theory – Chemistry Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2? Solution. We draw a molecular orbital energy diagram similar to that shown in Figure 11.
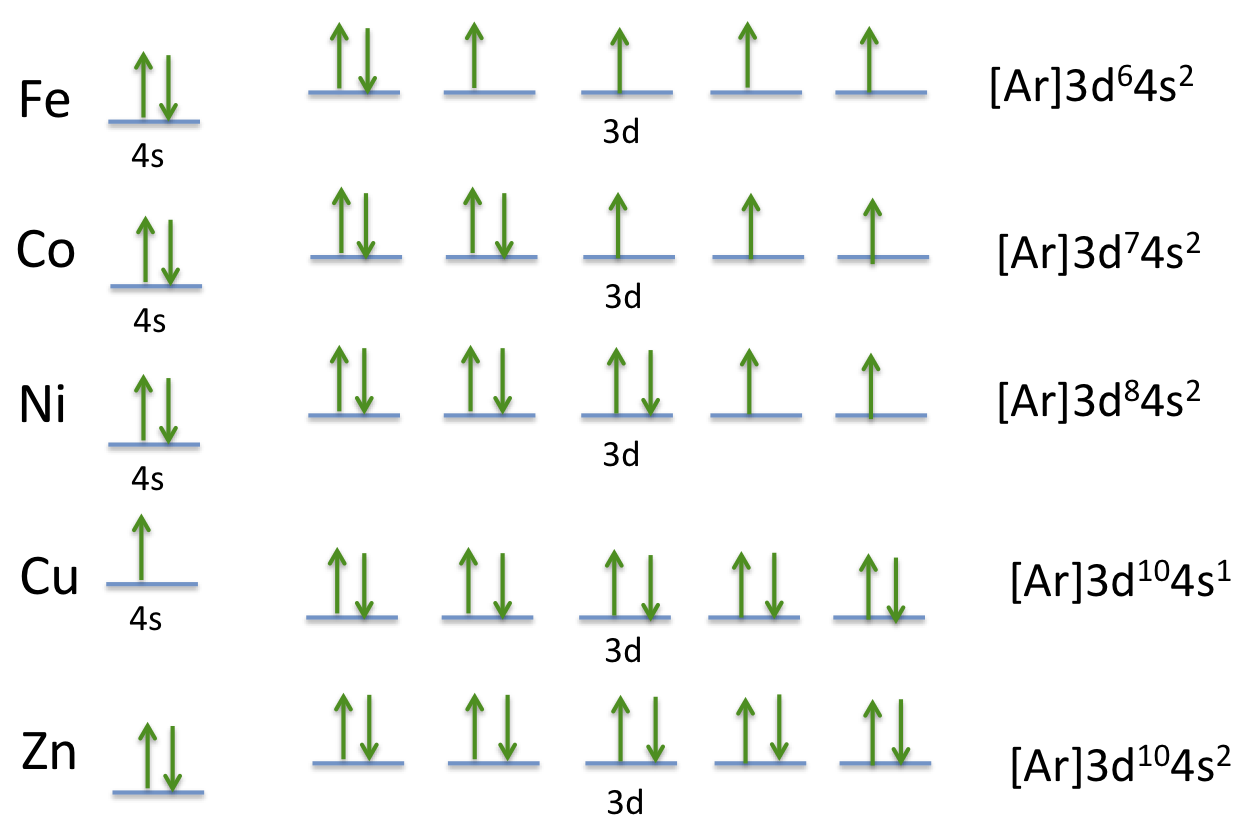
Orbital Diagram of All Elements (Diagrams given Inside) 10/04/2021 · Orbital diagram of Sulfur (S) 17: Orbital diagram of Chlorine (Cl) 18: Orbital diagram of Argon (Ar) 19: Orbital diagram of Potassium (K) 20: Orbital diagram of Calcium (Ca) 21: Orbital diagram of Scandium (Sc) 22: Orbital diagram of Titanium (Ti) 23: Orbital diagram of Vanadium (V) 24: Orbital diagram of Chromium (Cr) 25: Orbital diagram of Manganese … H2S Lewis Structure, Molecular Geometry, Hybridization ... 26/02/2022 · H2S Molecular Orbital (MO) Diagram. The molecular orbital diagram of H2S can be explained in the following way. This is the MO diagram of H2S. The left-hand side will contain the atomic orbitals of sulfur i.e 3s2 3px2 3py1 3pz1. And on the right-hand side, there will be atomic orbitals of hydrogen. 8 valence electrons are filled in the MO ... Electron Configuration for Sulfur (S) - TerpConnect In writing the electron configuration for Sulfur the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons ...Oct 24, 2016 · Uploaded by Wayne Breslyn The Electron Configurations of Atoms - Chemistry at Illinois The fifth electron is added to a 2p orbital, the sublevel next higher in energy (Figure 5.9). The electron configuration of boron is: B: 1s 2 2s 2 2p 1. Table 5.2 shows the electron configurations of the elements with atomic numbers 1 through 18. The electron configurations of elements with higher atomic number can be written by following the orbital-filling chart in Figure 5.9. TABLE …
Electron Configurations - Department of Chemistry ... Orbital Diagrams. Another way to represent the order of fill for an atom is by using an orbital diagram often referred to as "the little boxes": The boxes are used to represent the orbitals and to show the electrons placed in them. The order of fill is the same but as you can see from above the electrons are placed singly into the boxes before ...







![Electron Configuration | Chemistry [Master]](https://textimgs.s3.amazonaws.com/boundless-chemistry/gram-four-2p-hund-27s-rule.svg)
/Lewis-dot-58f78f405f9b581d5938e617.jpg)







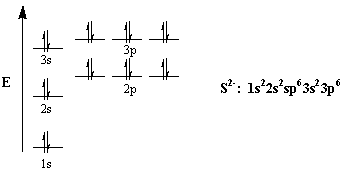




0 Response to "42 orbital filling diagram for sulfur"
Post a Comment