38 atomic energy level diagram
Atomic Energy Levels and Grotrian Diagrams | ScienceDirect Atomic Energy Levels and Grotrian Diagrams, Volume I: Hydrogen I - Phosphorus XV presents diagrams of various elements that show their energy level and electronic transitions. The book covers the first 15 elements according to their atomic number. Atomic Energy Levels (video) - Khan Academy We like representing these energy levels with an energy level diagram. The energy level diagram gives us a way to show what energy the electron has without having to draw an atom with a bunch of circles all the time. Let's say our pretend atom has electron energy levels of zero eV, four eV, six eV, and seven eV.
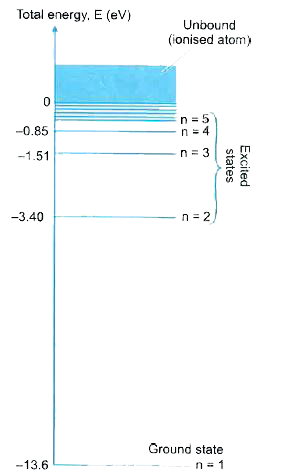
PhysicsLAB: Energy-Level Diagrams Using the Bohr Model, the energy levels (in electron volts, eV) are calculated with the formula: En = -13.6 (Z2/n2) eV where Z is the atomic number and n is the energy level. The ground state is represented by n = 1, first excited state by n = 2, second excited state by n = 3, etc. eV
Atomic energy level diagram
PDF Energy Level Diagram - Tripod Chemistry - Energy Level Diagrams . Title: Energy Level Diagram Author: HERH Created Date: 2/10/2005 1:01:43 PM ... Lithium(Li) electron configuration and orbital diagram The electron configuration of an element with an atomic number greater than 18 cannot be properly determined according to the Bohr atomic model. The electron configuration of all the elements can be done through the orbital diagram. Electron configuration of lithium atom through orbital. Atomic energy levels are subdivided into sub-energy levels. PDF Energy Level Diagram Chemistry - Energy Level Diagrams . Title: Energy Level Diagram Author: HERH Created Date: 2/10/2005 12:17:57 PM ...
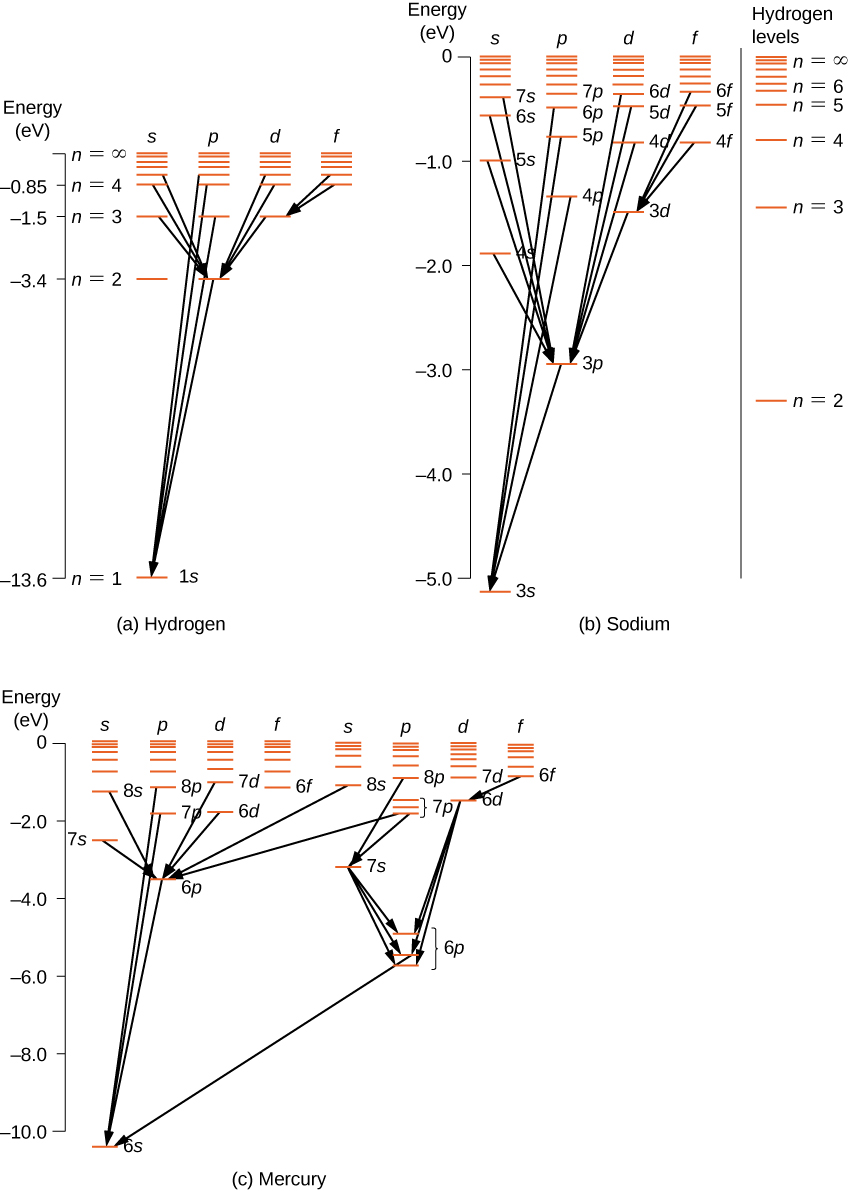
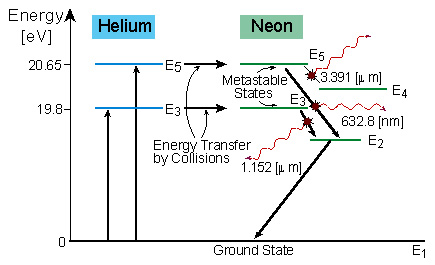
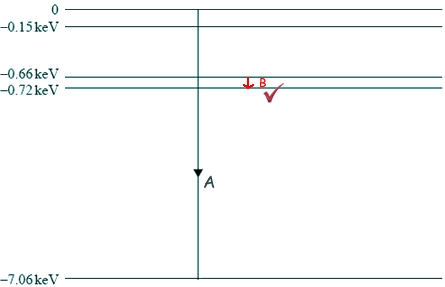
Atomic energy level diagram. Energy Levels of Hydrogen Atom (Energy Level Diagrams)। B ... Elements of Quantum Mechanics: Atomic and Molecular SpectraFor more videos follow the following links given below..👉For Atomic Energy Levels click on the li... PDF Figure 1. Mercury energy level diagram collision transfers energy greater than the ionization potential of the atom. Likewise an ionized atom can capture a free electron. All these events occur at different rates, depending in part on the conditions of the gas. Figure 1 shows the energy levels for one element (mercury). Figure 1. Mercury energy level diagram PDF Chapter 8 Atomic Absorption Spectrophotometry Energy Level Diagrams. Energy level diagram (Mg atom) Energy Level Diagrams for lower states of Na, Mg, Al. Ionic spectra versus atomic spectra ... • Atomic Absorption: it measures the radiation absorbed by the unexcited atoms that are determined. 1.4: Atomic Energy Levels Flashcards | Quizlet Atomic energy transformations can be represented using an energy level diagram--> think of a ball on a staircase: gain of energy = move up step/s, loss of energy = move down step/s, ΔE = change of energy (energy required/difference in energy)
Atomic energy levels and Grotrian diagrams. Vol. I ... A collation is presented of all present information concerning electronic transitions in monatomic systems. The data are presented in the form of energy-level diagrams and Grotrian diagrams (showing transitions from one spectroscopic term to another). This volume covers the elements H, He, Li, Be, B, C, N, O, F, Ne, Na, Mg, Al, Si and P. Authors: Atom Diagrams: Electron Configurations of the Elements For each electron shell atom diagram, the element symbol is listed in the nucleus. The electron shells are shown, moving outward from the nucleus. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. The element atomic number and name are listed in the upper left. Atomic Energy Level Diagrams - Georgia State University Atomic Energy Level Diagrams Energy level diagrams can be useful for visualizing the complex level structure of multi-electron atoms. Forms of such diagrams are called Grotrian diagrams or term diagrams in various parts of the literature. Lithium energy-level diagram - Big Chemical Encyclopedia Lithium energy-level diagram Energy level diagrams for the easily excited atomic lines of lithium, sodium, potassium and rubidium. Wavelengths are given in nanometres for the spectral lines produced by transitions between the different levels. The ionization potential is indicated by the dashed line above the respective diagrams. The Selection Rule for L - The energy-level diagram for lithium ...
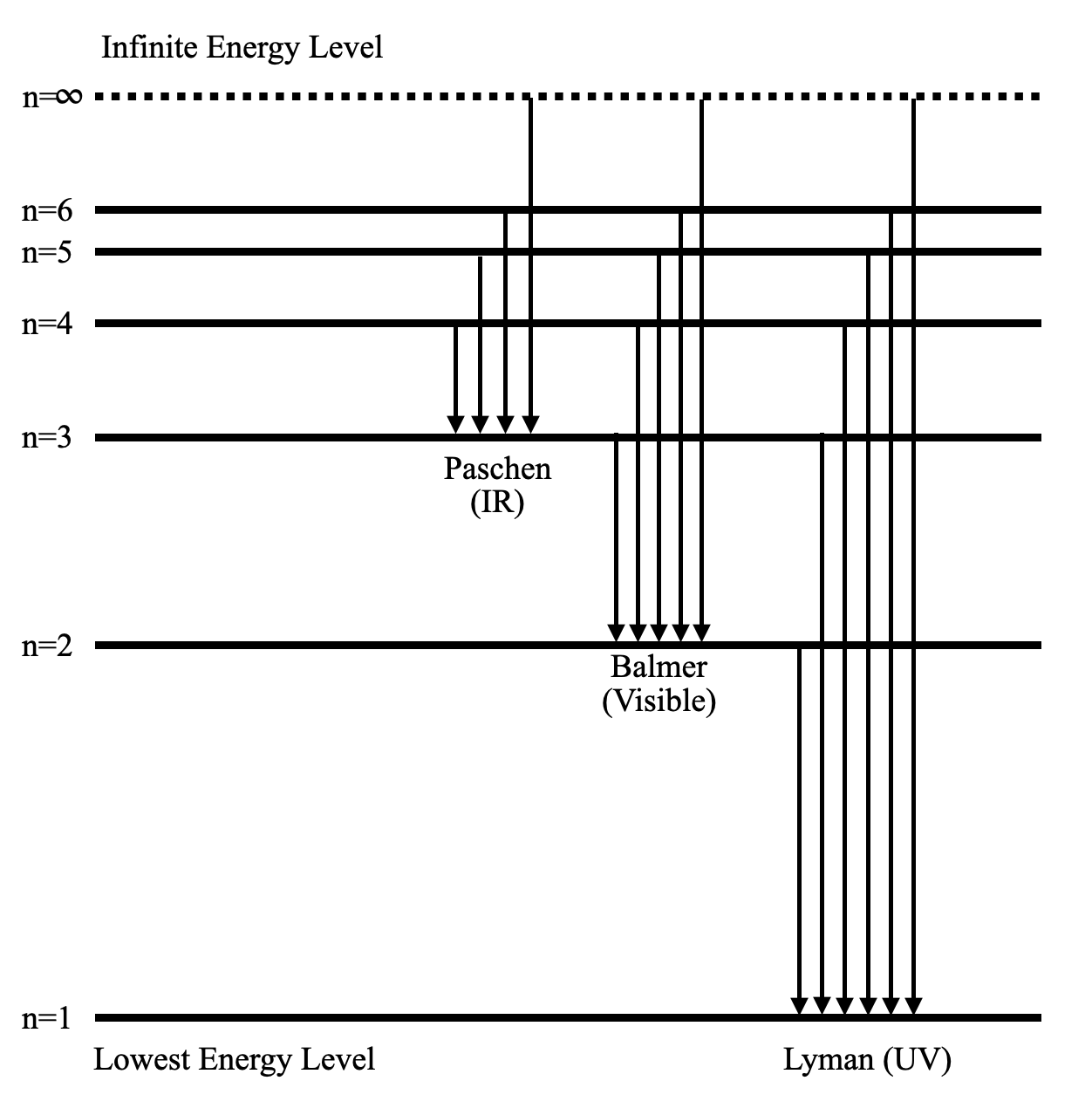
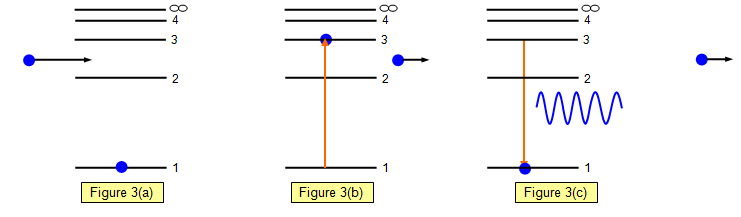
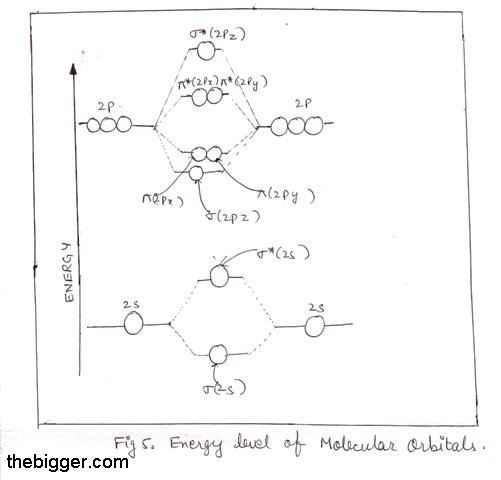
Lesson Worksheet:Electron Energy Levels | Nagwa Lesson Worksheet: Electron Energy Levels. In this worksheet, we will practice determining whether an electron shell of an atom is filled and which electron transitions are possible in a given atom. The diagram shows a hydrogen atom. The electron shown transitions between two energy levels of the atom. tikz pgf - Energy level diagrams with TeX - TeX - LaTeX ... Examples for Energy level diagrams Links: ... All certainly doable (see for example the modiagram package, which does atomic/molecular orbitals), but 'easy to handle' will depend on your requirements. Perhaps you could illustrate what sort of input you are hoping to use so we can gauge how easy this might be. Energy level diagram for Molecular orbitals - Chemical ... Energy level diagram for Molecular orbitals Last Updated on March 20, 2019 By Mrs Shilpi Nagpal 18 Comments Energy level diagram for Molecular orbitals The first ten molecular orbitals may be arranged in order of energy as follow: σ (1s) <σ∗(1s) < σ (2s) <σ∗(2s) < π (2px) = π (2py) < σ (2pz) < π∗(2px) =π∗(2py) <π∗( 2pz) PDF Atomic Energy-Level Diagrams - University of Birmingham transitions to a higher energy level by absorption of a photon or in a collision between atoms; they can make transitions to lower energy levels by emission of a photon. On the energy-level diagram for hydrogen, the n =∞level has an energy of 13.6 eV. If the electron is initially in the ground state and the atom absorbs a photon of any energy
Energy Level Diagram: Properties, Energy Shells, Principles The Energy Level, known popularly as the electron shell, is the orbit with electrons that surround the nucleus of an atom. As per definition, the ascending order arrangement of energies in an orbital is called Energy Level Diagram. The alignment around the atom occurs in consecutive shells, named as L shell, M shell, and so on.
Energy Level - Principal Quantum Number | Bohr's Atomic ... This model is popularly known as the Bohr model of an atom. Bohr proposed an atomic model of a hydrogen atom. Bohr's model gave a proper explanation for the stability of electrons revolving in orbits. He named these orbits as energy shells. His postulates explaining the energy level are discussed below. Bohr's Explanation for Energy Level:
Energy Level Diagrams - Chemistry LibreTexts At sufficiently high energy, an electron is removed and the atom ionized. The ion has widely spaced energy levels, that in turn get closer and closer together until another electron can be removed. This process can continue until all the electrons are ionized and the nucleus is bare. The only common fully-ionized species is H ion, a bare proton.
NIST: Atomic Spectra Database - Energy Levels Form NIST Atomic Spectra Database Levels Form. This form provides access to NIST critically evaluated data on atomic energy levels. Spectrum: e.g., Fe I or Mg Li-like or Z=59 II or 198Hg I.
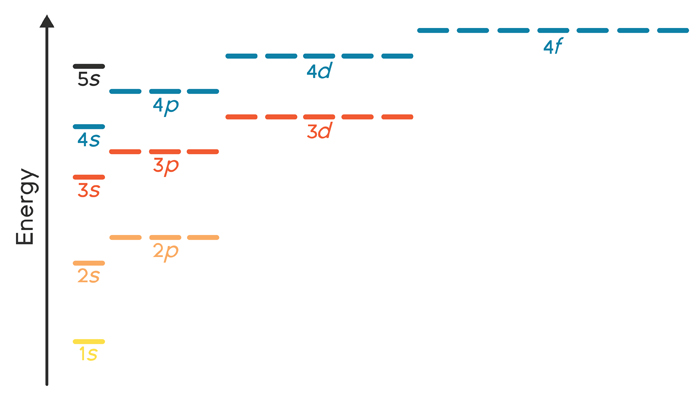
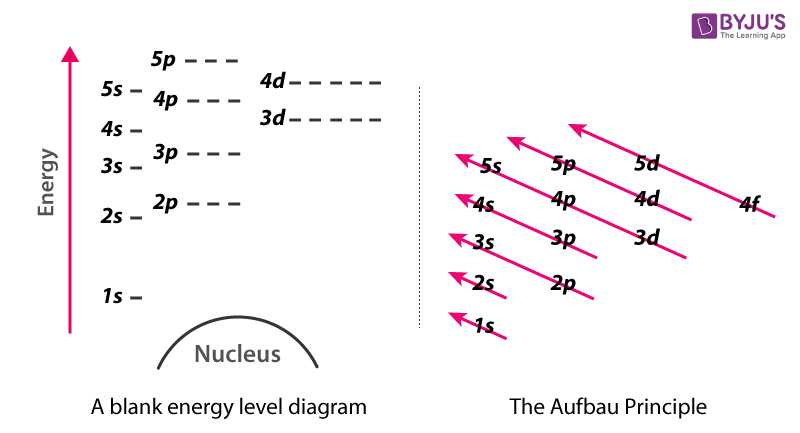
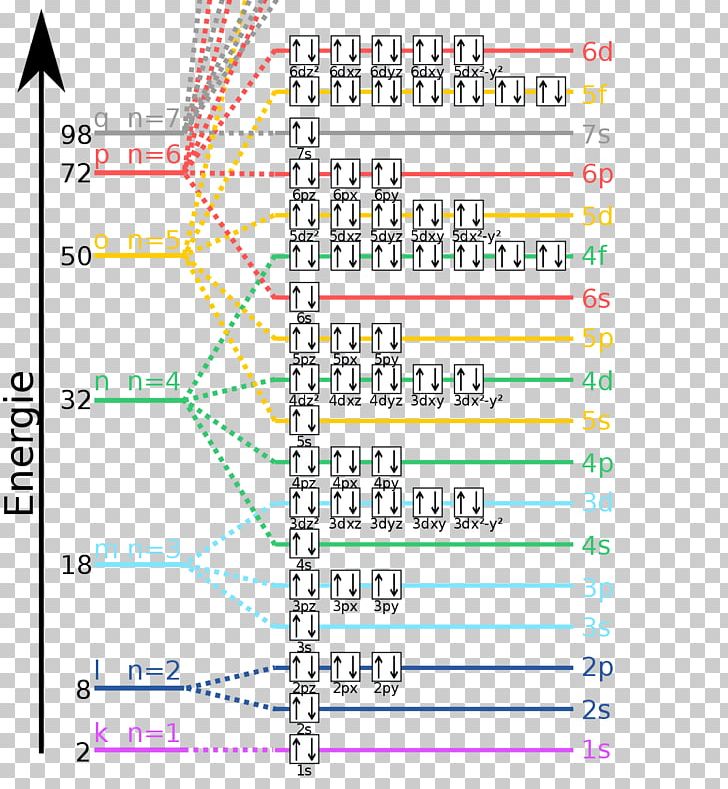
Energy Level Diagram - Different Energy Shells Around ... Below is a blank energy level diagram which helps you depict electrons for any specific atom. At energy level 2, there are both s and p orbitals. The 2s has lower energy when compared to 2p. The three dashes in 2p subshells represent the same energy. 4s has lower energy when compared to 3d. Therefore, the order of energy level is as follows:
plotting - Plot an energy level diagram - Mathematica ... Plot an energy level diagram. Ask Question Asked 1 year, 10 months ago. Modified 1 year, 10 months ago. Viewed 737 times 3 2 $\begingroup$ I have the following list: ... Energy Levels. 7. How to draw ranges in a plot? 0. Drawing a diagram showing how a parabola is generated. 0.
PDF Energy Level Diagrams - Ms. Scott's Site Energy Level Diagrams Atomic Number Atomic # represents the number of protons (p+) in the nucleus of an atom. For a neutral atom the # protons = # electrons. (total positive charge equals the total negative charge). The atomic number is the top right number on the periodic table for each atom. Example: Carbon has atomic # 6 which means C has
Atomic energy-level and Grotrian diagrams. Volume III ... @misc{etde_5286863, title = {Atomic energy-level and Grotrian diagrams. Volume III. Vanadium I - Chromium XXIV} author = {Bashkin, S, and Stoner, Jr, J O} abstractNote = {This monograph presents diagrammatically the spectra of atoms and monatomic ions in the range vanadium I to chromium XXIV. All observed transitions and all known levels are included. Two decimal digits have been kept for most ...
Energy level - Wikipedia The molecular energy levels are labelled by the molecular term symbols. The specific energies of these components vary with the specific energy state and the substance. Energy level diagrams. There are various types of energy level diagrams for bonds between atoms in a molecule. Examples
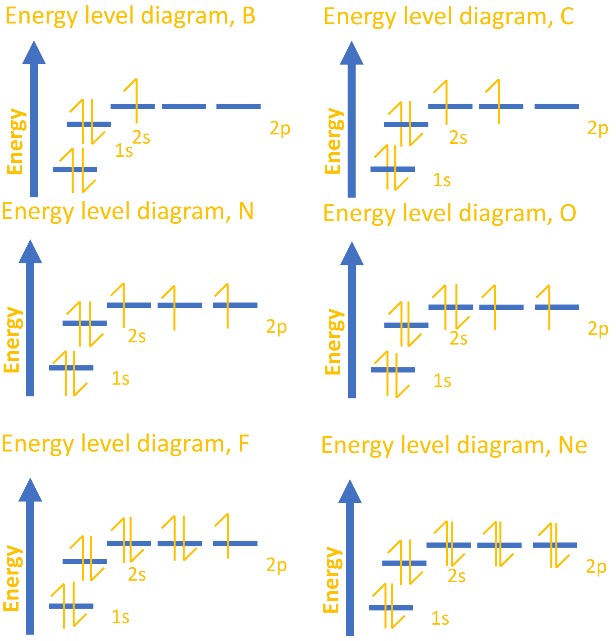
Hydrogen(H) electron configuration and orbital diagram Atomic energy levels are subdivided into sub-energy levels. These sub-energy levels are called orbital. The sub energy levels are expressed by 'l'. The value of 'l' is from 0 to (n - 1). The sub-energy levels are known as s, p, d, f. Determining the value of 'l' for different energy levels is- If n = 1, (n - 1) = (1-1) = 0
PDF Orbital Energy vs. Atomic Number - University of Illinois ... Li Na electron configurations with energy minima Based on the previous graph, this schematic diagram shows how the energy of the highest filled atomic level varies with atomic number (see previous figure). The energy minima at He, Ne, and Ar are spaced apart by eightatomic numbers. 8 steps Period 2 Orbital Energies of Neutral Atoms & Ions
PDF Energy Level Diagram Chemistry - Energy Level Diagrams . Title: Energy Level Diagram Author: HERH Created Date: 2/10/2005 12:17:57 PM ...
Lithium(Li) electron configuration and orbital diagram The electron configuration of an element with an atomic number greater than 18 cannot be properly determined according to the Bohr atomic model. The electron configuration of all the elements can be done through the orbital diagram. Electron configuration of lithium atom through orbital. Atomic energy levels are subdivided into sub-energy levels.
PDF Energy Level Diagram - Tripod Chemistry - Energy Level Diagrams . Title: Energy Level Diagram Author: HERH Created Date: 2/10/2005 1:01:43 PM ...












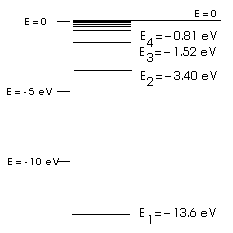








0 Response to "38 atomic energy level diagram"
Post a Comment