38 show the orbital-filling diagram for n (nitrogen)
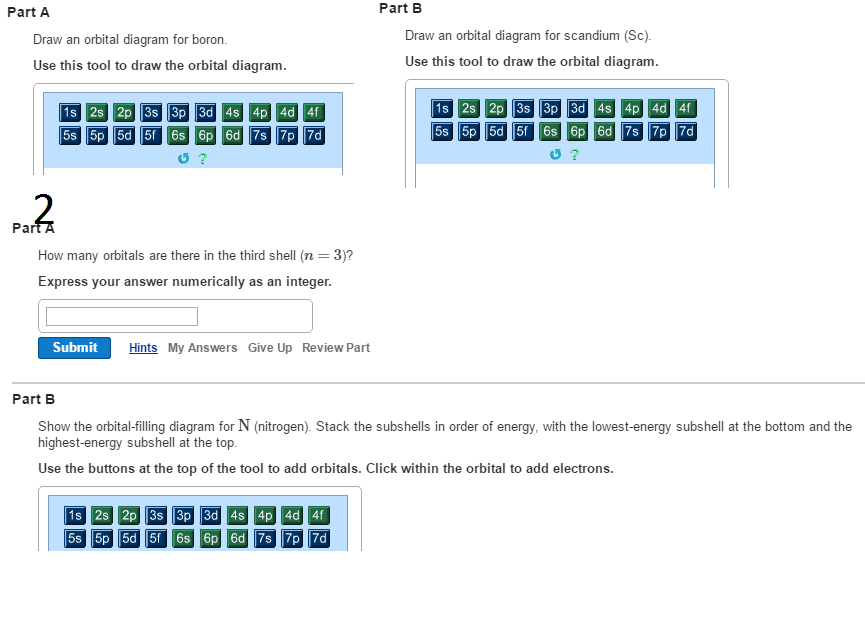
Chemistry Atomic concepts Flashcards | Quizlet Show the orbital-filling diagram for N (nitrogen). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Show the orbital-filling diagram for S (sulfur). part A;Show the orbital-filling diagram for N (nitrogen ... help Show the orbital-filling diagram for N (nitrogen). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Use the buttons at the top of the tool to add orbitals. Click within the orbital to add electrons. Show the orbital-filling diagram for S (sulfur).
Solved Show the orbital-filling diagram for N (nitrogen ... Show the orbital-filling diagram for N (nitrogen). Order subshells by energy, with the lowest-energy subshell at the left Drag the appropriate labels to their respective targets. View Available Hint(s) Reset Help 11 || 1 18 2s 2p 3s ap Group 1 GT G1 G1 G1 GI GT G161 G2 G2 G2 G2 G2 ; Question: Show the orbital-filling diagram for N (nitrogen ...

Show the orbital-filling diagram for n (nitrogen)
Nitrogen(N) electron configuration and orbital diagram Orbital Diagram for Nitrogen (N) Nitrogen (N) excited state electron configuration Atoms can jump from one orbital to another by excited state. This is called quantum jump. Ground state electron configuration of nitrogen is 1s 2 2s 2 2p 3. The p-orbital has three sub-orbitals. The sub-orbitals are p x, p y, and p z. Molecular orbital energy level diagrams -Hydrogen ... The molecular orbital energy level diagram of N 2 is given in Fig.. The bond order of N 2 can be calculated as follows. Here, N b = 8 and N a = 2 . ∴ Bond order = (N b -N a ) / 2 =(8-2) /2 = 3. i. Nature of bond : A bond order of 3 means that a triple bond is present in a molecule of nitrogen. ii. Orbital Filling Diagram For Nitrogen Figure 1. The 2p .Show transcribed image text Show the orbital-filling diagram for N (nitrogen). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Use the buttons at the top of the tool to add orbitals. Click within the orbital to add electrons.
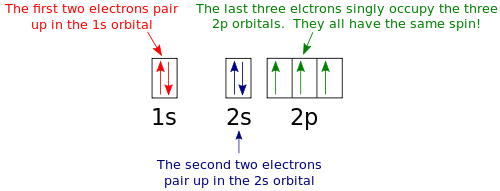
Show the orbital-filling diagram for n (nitrogen). Nitrogen (N) Orbital diagram, Electron configuration, and ... The orbital diagram for nitrogen is drawn with 3 orbitals. The orbitals are 1s, 2s, and 2p. The nitrogen orbital diagram contains 2 electrons in 1s orbital, 2 electrons in 2s orbital, and the rest three electrons in 2p orbital. The orbital diagram for a ground-state electron configuration of nitrogen atom is as follow- Orbital Filling Diagram For Bromine The orbital diagram for bromine shows four concentric circles around a dot representing a nucleus, with two dots on the first circle, eight on the second, 18 on the third and seven on the fourth. Each dot on a circle represents an electron. Periodic Trends. STUDY. PLAY. Item 1: Part A Show the orbital-filling diagram for N (nitrogen). Orbital Filling Diagram For Sulfur The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each. The arrows represent the 16 electrons of the sulfur atom, and the directions represent their spins. The boxes represent sulfur's orbitals. Sulfur's electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 4. Hund's Rule and Orbital Filling Diagrams | Chemistry for ... In an orbital filling diagram, the individual orbitals are shown as circles (or squares) and orbitals within a sublevel are drawn next to each other horizontally. Each sublevel is labeled by its principal energy level and sublevel. Electrons are indicated by arrows inside the circles.
Show the orbital filling diagram for Nitrogen? | Study.com Show the orbital filling diagram for Nitrogen? Electronic configuration: The filling of an electron in an orderwise manner in the orbital of an atom or element is called the electronic configuration. Orbital Diagram For Nitrogen (N) | Nitrogen Electron ... Nitrogen is an element that has 7 electrons and when we talk about the valence electrons then, valence electrons are those electrons that are present in the outer shell and are associated with molecules or an atom and which also can participate in the chemical formation. Show The Orbital Filling Diagram For Sulfur - schematron.org Show the orbital-filling diagram for (bromine).Status: Resolved. In writing the electron configuration for Sulfur the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for sulfur go in the 2s orbital. The next six electrons will go in the 2p orbital. Orbital filling diagrams - The Cavalcade o' Chemistry The orbital filling diagram for carbon Again, we start with the electron configuration, which is 1s²2s²2p². As we've seen, this means that there are 2 electrons in the 1s orbital, two electrons in the 2s orbital, and two electrons in the 2p orbitals. This is shown like this:
An orbital can hold up to two electrons which must have ... Click within the orbital to add electrons. ANSWER: View Correct Part D Show the orbital-filling diagram for (bromine). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Answered: Show the orbital-filling diagram for N… | bartleby Part B Show the orbital-filling diagram for N (nitrogen). Order subshells by energy, with the lowest-energy subshell at the left. Drag the appropriate labels to their respective targets. > View Available Hint (s) Reset Help 1L 1s 2s 2p 3s 3p G1 G1 G1 G1 G1 G1 G1 G1 G1 G2 G2 G2 G2 G2 Submit Part C MacBook Air Expert Solution Show The Orbital Filling Diagram For Br Bromine Item 1: Part A Show the orbital-filling diagram for N (nitrogen). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Show the orbital-filling diagram for Br (bromine). Stack the subshells in order of energy, with the lowest. May 01, · I think I did everything right. Orbital Filling Diagram For Nitrogen - schematron.org If that single electron were a spin-up (ms = +1/2), the orbital diagram for The figure below illustrating orbital diagrams for nitrogen is similar to the. Use orbital filling diagrams to describe the locations of electrons in an atom. Diagram of Hund's rule in boron, carbon, nitrogen, and oxygen. Figure 1. The 2p .
Show the orbital-filling diagram for N (nitrogen ... Show the orbital-filling diagram for N (nitrogen). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top.1s, 2s, 2p, 3s, 3p, 3d, 4s, 4p, 4d, 4f, 5s, 5p, 5d, 5f, 6s, 6p, 6d,7s, 7p, 7d3. Show the orbital-filling diagram for S (sulfur).
Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagram of Helium (He) 3: Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12 ...
(Solved) - Part A How many orbitals are there in the third ... Express your answer numerically as an integer. Part B Show the orbital-filling diagram for N (nitrogen). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Part C Show the orbital-filling diagram for S (sulfur).
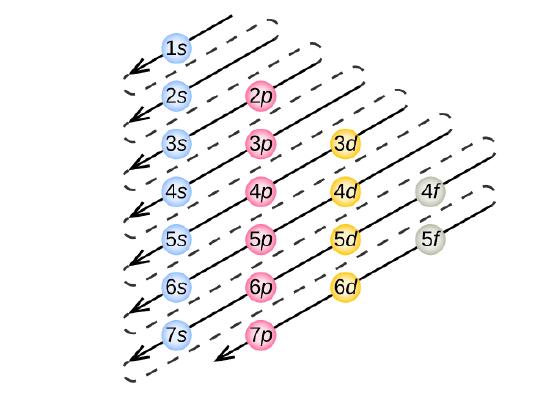
How to Do Orbital Diagrams - Sciencing The Aufbau principle tells you that the lowest-energy orbitals fill first, but the specific order isn't sequential in a way that's easy to memorize. See Resources for a diagram showing the filling order. Note that the n = 1 level only has s orbitals, the n = 2 level only has s and p orbitals, and the n = 3 level only has s, p and d orbitals.
How many electrons are unpaired in the orbitals of nitrogen? 3 Unpaired electrons. Nitrogen atom has total 7 electrons. Two will fill up the n=1 level, and then there are five electrons in the n=2 level. Nitrogen can bond three times with other electrons to fill up it's shell with 8, (8-5=3). And these are those 3 unpaired electrons which were residing the 2p sub-shell of the Nitrogen atom , before the formation of 3 bonds.
Periodic Trends Flashcards - Quizlet Show the orbital-filling diagram for N (nitrogen). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. 1s²2s²2p³ Item 2: Part C Show the orbital-filling diagram for S (sulfur).
Answered: Show the orbital-filling diagram for N… | bartleby Show the orbital-filling diagram for N (nitrogen). Order subshells by energy, with the lowest-energy subshell at the left. Drag the appropriate labels to their respective targets. View Available Hint(s) Reset Help 1L 1 1s 2s 2p 3s Зр G1 G1 G1 G1 G1 G1 G1 G1 G1 G2 G2 G2 G2 G2 Part C Show the orbital-filling diagram for S (sulfur).
Electron Configuration for Nitrogen (N) - UMD Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for N goes in the 2s orbital. The remaining three electrons will go in the 2p orbital. Therefore the N electron configuration ...
Solved Show the orbital-filling diagram for N (nitrogen ... Expert Answer 100% (19 ratings) Transcribed image text: Show the orbital-filling diagram for N (nitrogen). Order subshells by energy, with the lowest-energy subshell at the left. Drag the appropriate labels to their respective targets.
OneClass: Part C Show the orbital-filling diagram for S ... Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top.i put 1s,2s,2p,3s,3p and still said wrong 3) Show the orbital-filling diagram for (nitrogen).
Orbital Filling Diagram For Nitrogen Figure 1. The 2p .Show transcribed image text Show the orbital-filling diagram for N (nitrogen). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Use the buttons at the top of the tool to add orbitals. Click within the orbital to add electrons.
Molecular orbital energy level diagrams -Hydrogen ... The molecular orbital energy level diagram of N 2 is given in Fig.. The bond order of N 2 can be calculated as follows. Here, N b = 8 and N a = 2 . ∴ Bond order = (N b -N a ) / 2 =(8-2) /2 = 3. i. Nature of bond : A bond order of 3 means that a triple bond is present in a molecule of nitrogen. ii.
Nitrogen(N) electron configuration and orbital diagram Orbital Diagram for Nitrogen (N) Nitrogen (N) excited state electron configuration Atoms can jump from one orbital to another by excited state. This is called quantum jump. Ground state electron configuration of nitrogen is 1s 2 2s 2 2p 3. The p-orbital has three sub-orbitals. The sub-orbitals are p x, p y, and p z.
























0 Response to "38 show the orbital-filling diagram for n (nitrogen)"
Post a Comment