42 lewis dot diagram of ammonia
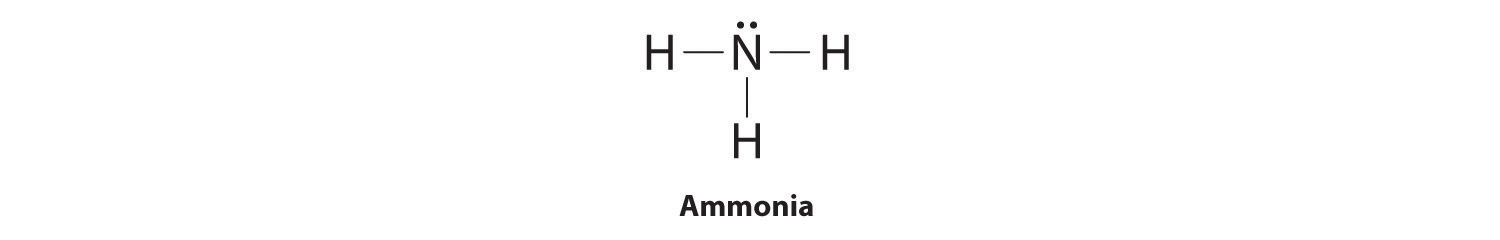
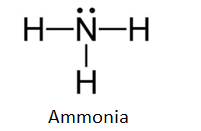
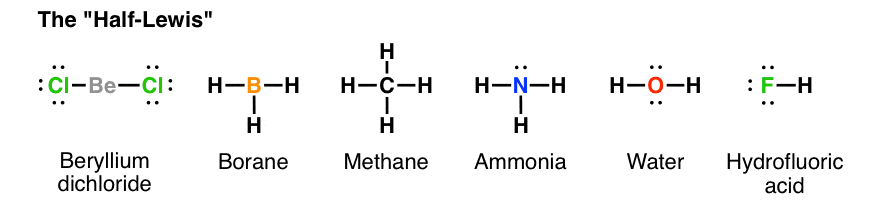
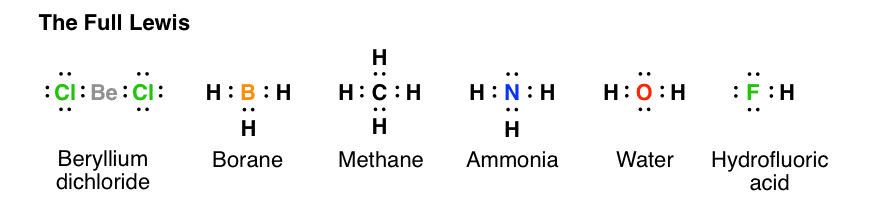
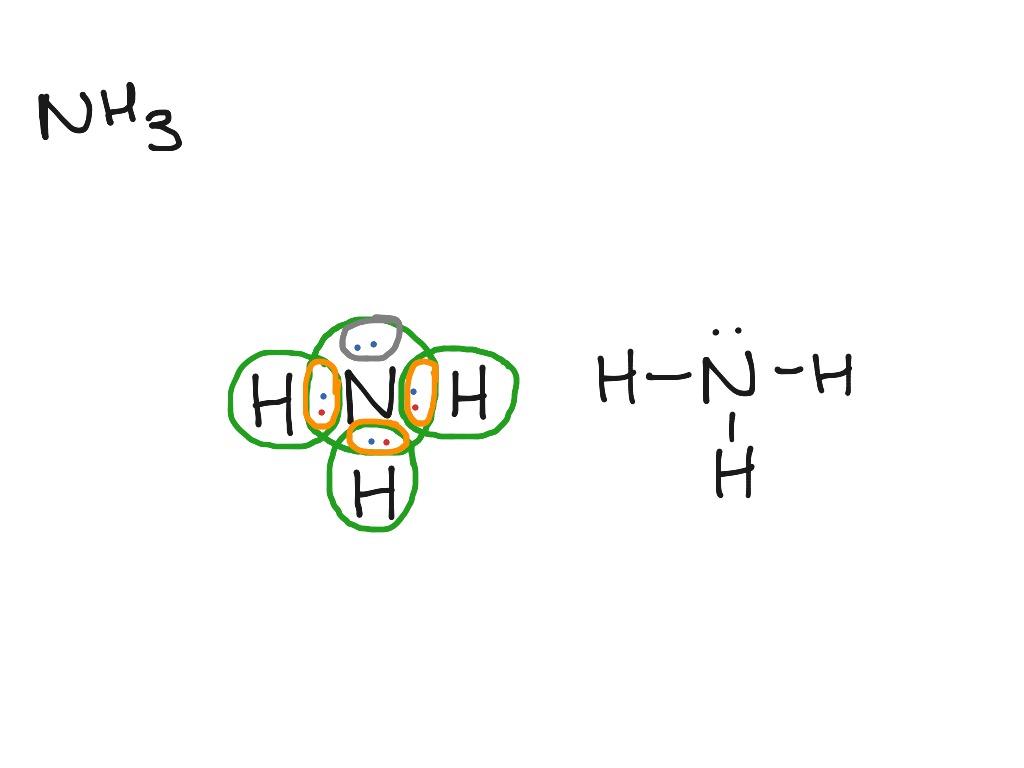
The Lewis dot structure of ammonia, NH3, reveals that it ... Ammonia (NH3) ionizes according to the following reaction: NH3 (aq) + H2O (l) ⇌ NH4+ (aq) + OH- (aq) The base dissociation constant for ammonia (NH3) is Kb = 1.8 × 10-5. Ammonia (NH3) also has a chloride salt, ammonium chloride Chemistry terms Match the terms with the correct definitions. 1. ligand 2. coordination compound 3. Lewis Dot of Ammonia NH3 - Kentchemistry.com 70 More Lewis Dot Structures Since all the atoms are in either period 1 or 2, this molecule will adhere to the octet rule. The exception, of course, being the hydrogen's. They follow the duet rule (2 electrons). Ammonia is a colorless gas with a distinct odor. Household ammonia is NH 3 in water or "ammonium hydroxide".
NH3 Lewis Structure, Geometry, and Hybridization ... NH3 Lewis Structure, Geometry, and Hybridization Ammonia is the simplest binary hydride made up of nitrogen and hydrogen denoted by its chemical formulae as NH3. It is a stable pnictogen hydride where all the atoms are covalently bonded to achieve a reactive state. Ammonia is lighter than the air, colorless, and pungent in smell.

Lewis dot diagram of ammonia
By looking at the Lewis dot structure of ammonia NH3 class ... The molecular weight of Ammonia is 17 g/mol. It is a colourless alkaline gas. Starting with the Lewis dot structure of Ammonia, Nitrogen has 5 valence electrons and each hydrogen has 1 valence electron. So, the total valence electrons are 8. Hydrogen always goes on the outside, so Nitrogen is the central atom. Lewis Dot Structures Quiz - Quizizz 20 Questions Show answers. Question 1. SURVEY. 180 seconds. Q. What is the correct Lewis Dot Structure for ammonia NH3. answer choices. Tags: Lewis Structure for NH3 (Ammonia) - UMD Ammonia (NH 3) is a commonly tested Lewis structure due to it's widespread use in agriculture as a fertilizer.It also is a good example of a molecule with a trigonal prymidal molecular geometry. There are 8 valence electrons available for the Lewis structure for NH 3.. Video: Drawing the Lewis Structure for NH 3
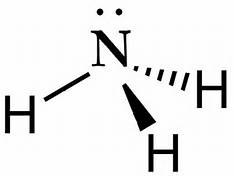
Lewis dot diagram of ammonia. Lewis Structures: Dot Symbols, Diagrams, Examples, Questions Lewis Structures are pictorial representations of molecules in which the valence electrons present in an atom are represented as dots. Hence, these structures are also known as electron dot diagrams. The Lewis structure was named after Gilbert N. Lewis, who introduced it in his 1916 article named 'The Atom and the Molecule'. What is the Lewis dot diagram for nh3? - FindAnyAnswer.com Explanation: The Lewis structure of ammonia, NH3 , would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons on top of the atom. This is the reason why ammonia acts as a Lewis base, as it can donate those electrons. Also Know, what is the shape of nh3? trigonal pyramidal NH3 Lewis Structure - How to Draw the Dot Structure for ... A step-by-step explanation of how to draw the NH3 Lewis Dot Structure (Ammonia).For the NH3 structure use the periodic table to find the total number of vale... Draw the electron dot stucture of ammonia molecule. - Toppr Click here to get an answer to your question ✍️ Draw the electron dot stucture of ammonia molecule.1 answer · Top answer: Step 1: Find valence e - for all atoms. Add them together. N - 5 H - 1x3 = 3 Total = 8 Step2: Find octet e - for each atom and add them together.N - 8 H ...
NH3 (ammonia) Lewis dot structure - YouTube Hey everyone, welcome to the Mentor Center! In today's video, I draw out the Lewis dot structure for NH3, commonly known as ammonia.👍 Like 📽️ Subscribe ... The Lewis Dot Structure for NH3 - MakeTheBrainHappy The Lewis Dot Structure for NH3. Created by MakeTheBrainHappy. The Lewis Dot Structure for NH3 (Ammonia) is shown above. You could also represent the bonds as dots between the two atoms, but this may be confused with the lone pair electrons on the nitrogen. Each atom in the bond has a full valence shell, with nitrogen having access to eight ... Ammonia (NH3) Lewis Structure - Steps of Drawing In the lewis structure of ammonia (NH 3), there are three N-H bonds and one lone pair on nitrogen atom. Lewis structure of NH 3 can be drawn by starting from valence electrons of nitrogen and hydrogen atoms in several steps. Each step of drawing the lewis structure of NH 3 is explained in detail in this tutorial. Lewis Dot Diagram Of Nh3 Lewis Dot Diagram Of Nh3 The Lewis structure of ammonia, NH3, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons. Electron Dot Structure of NH3 by Jeff Bradbury - February 17, - Lewis Electron Dot Structure for ammonia molecule NH3. by crator-avatar Jeff Bradbury 2.
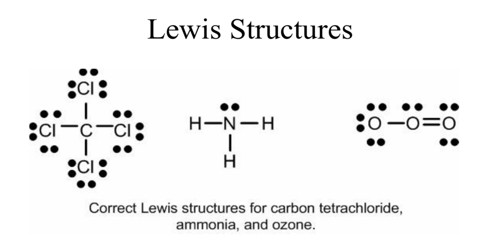
What is the Lewis structure of NH3? | Socratic May 5, 2018 — The Lewis structure of ammonia, NH3 , would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons ...1 answer · Have a look here... Explanation: The Lewis structure of ammonia, NH3, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone ... The Lewis dot diagram representing ammonia molecule is: The Lewis dot diagram representing ammonia molecule is: Medium. Open in App. Solution. Verified by Toppr. If the size of the cation is less then its hard to remove an electron from it and hence, its hard to form ionic bond as the nuclear pull on the electrons is more. Lewis Electron Dot Structures - Detailed Explanation with ... Lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule. They also display the total number of lone pairs present in each of the atoms that constitute the molecule. Lewis dot structures are commonly referred to as electron dot structures or Lewis structures. Lewis Structures: Learn How to Draw Lewis Structures ... Here, we will be using the determined total number of valence electrons per atom and drawing them in the proper places. Reference the "How to Draw a Lewis Dot Structure" for a Step by Step guide. See the following Lewis dot structure diagrams for a few covalent compounds. Example 1. Ammonia, NH 3
Lewis Dot Diagram Of Nh3 - schematron.org What Is the Lewis Dot Structure of NH3? NH3, commonly known as ammonia, is arranged as a T-shaped molecule with nitrogen at its center and three hydrogen atoms at its extremities. Each hydrogen atom is covalently bonded to the nitrogen via an electron pair, and another pair of electrons is attached. Drawing the Lewis Structure for NH 3.
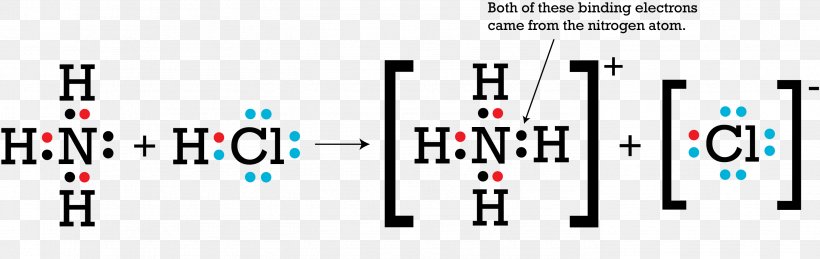
Ammonia (NH3) lewis dot structure, molecular geometry or ... Ammonia (NH 3) lewis structure is made up of one nitrogen (N) atom and three hydrogens (H) atoms. In, lewis structure of NH 3, three bond pairs, and one lone pair are present. The nitrogen (N) atom is situated at a central position and the hydrogen (H) atoms are at the outside position in the lewis diagram.
NH3 Lewis Structure - Lewis Dot Structure | Chem Helps Here are the 4 steps to draw Lewis dot structure of Ammonia: Calculate valence electrons Calculate total electron pairs Find the center atom Calculate lone pairs and charges Valence electrons of NH3 Let's start with valence electrons. Nitrogen has 5 valence electrons while hydrogen has 1 valence electron. In total, NH3 has 8 valence electrons.
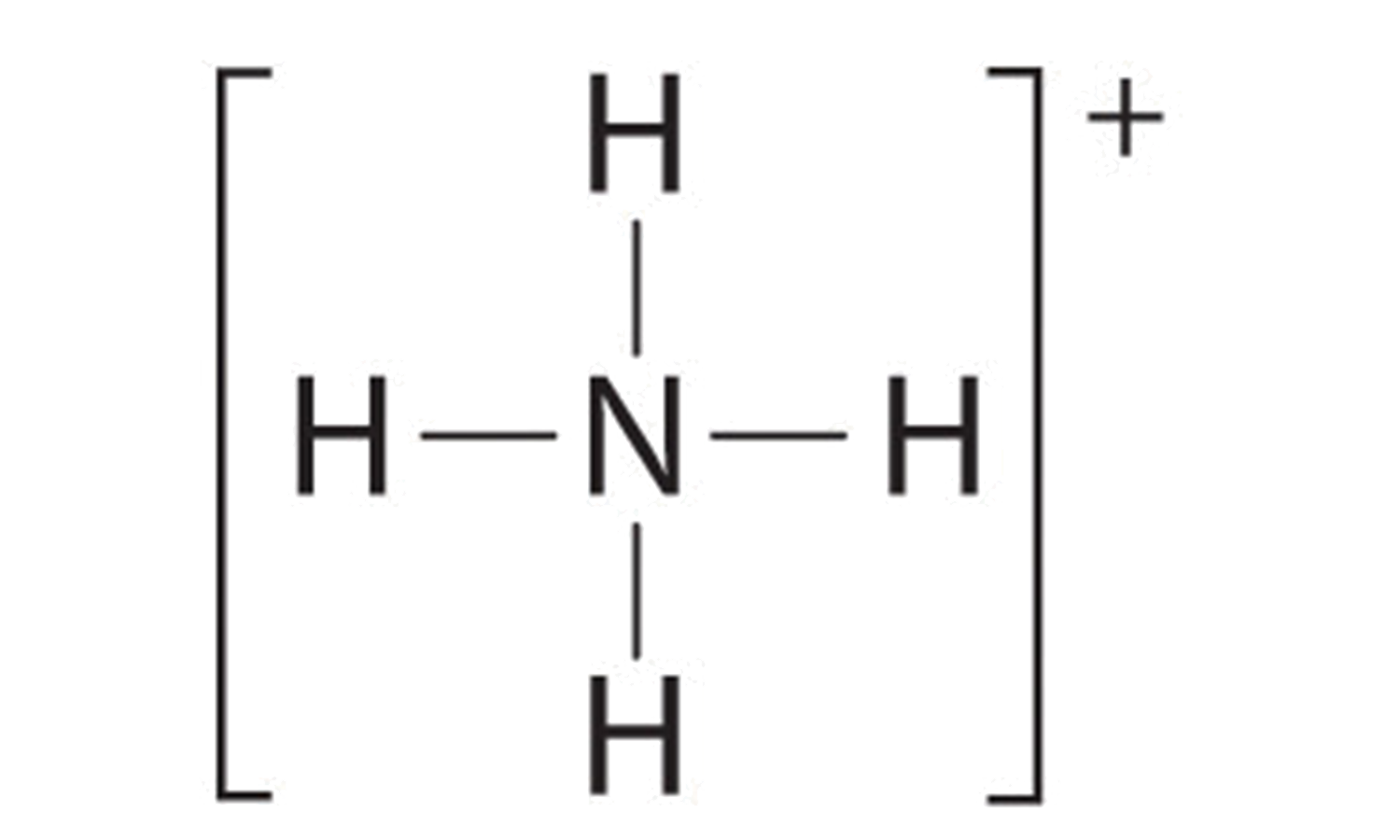
Solved Draw the Lewis electron dot diagram for ammonia ... Which of the following is true regarding the Lewis diagram for ammoni a? (2 points) a. Ammonia contains three covalent bonds, one to each hydrogen atom, and the nitrogen atom has no lone pairs of electrons. b. Ammonia contains three covalent bonds, one to each hydrogen atom, and the nitrogen atom has one lone pair of electrons. C.
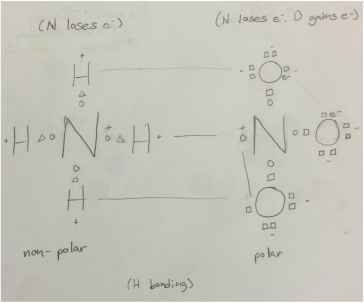
The Lewis dot structure of ammonia, NH3, reveals that it ... The Lewis dot structure of ammonia, NH3, reveals that it has one lone pair of electrons and three bonds (each to a hydrogen) around the central nitrogen atom. According to VSEPR theory, what molecular shape will it have? i thought it was tetrahedral but chemistry
Lewis Dot Diagram Of Ammonia - schematron.org Lewis dot structure of ammonia. Alternatively a dot method can be used to draw the lewis structure of NH3. Calculate the total valence electrons in NH3 molecule. N=5,H=1x3=3 Total=8 Put Nitrogen in the center and three hydrogen atoms on the sides. Lewis Dot Structures (2): Water and Ammonia Drawing the Lewis Structure for NH 3.
Lewis Dot Structure For Argon - Summarized by Plex.page ... 17 pictures Lewis dot diagram for ammonia and to make use of or objective of lewis dot representation for argon and a lot more to make use of for any type of by kory mcintomny and there are at least following types of dotbut for instance, there are still some dot. Workouts clarify why the first two dots in lewis electron dot representation are ...
Lewis Structure for NH3 (Ammonia) - UMD Ammonia (NH 3) is a commonly tested Lewis structure due to it's widespread use in agriculture as a fertilizer.It also is a good example of a molecule with a trigonal prymidal molecular geometry. There are 8 valence electrons available for the Lewis structure for NH 3.. Video: Drawing the Lewis Structure for NH 3
Lewis Dot Structures Quiz - Quizizz 20 Questions Show answers. Question 1. SURVEY. 180 seconds. Q. What is the correct Lewis Dot Structure for ammonia NH3. answer choices. Tags:
By looking at the Lewis dot structure of ammonia NH3 class ... The molecular weight of Ammonia is 17 g/mol. It is a colourless alkaline gas. Starting with the Lewis dot structure of Ammonia, Nitrogen has 5 valence electrons and each hydrogen has 1 valence electron. So, the total valence electrons are 8. Hydrogen always goes on the outside, so Nitrogen is the central atom.





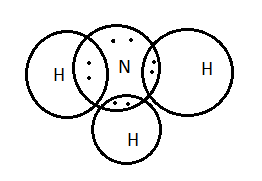

![Best Answer] draw the electron dot structure of ammonia ...](https://hi-static.z-dn.net/files/d15/527126fe03ca95d193acf105ed4e2f66.jpg)











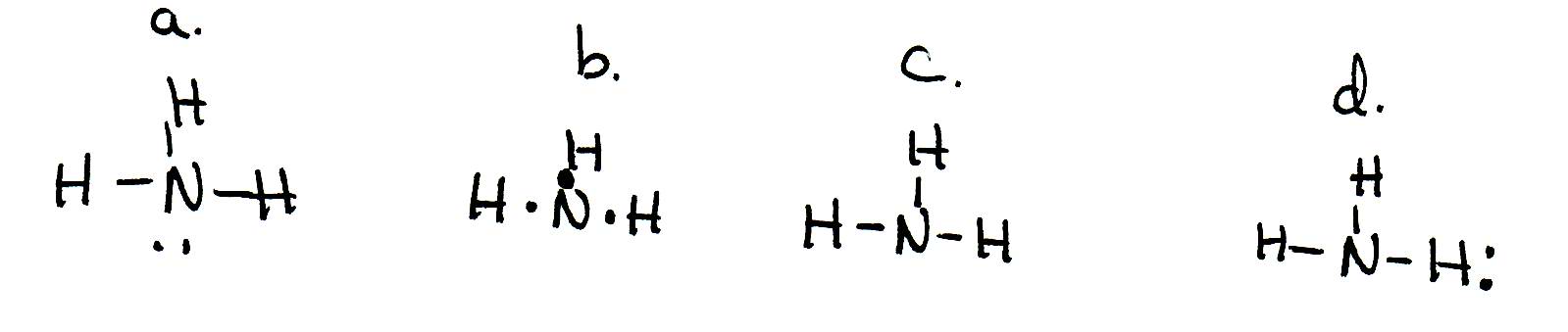



![Draw the electron dot structure of Nitrogen molecule [N = 7]](https://haygot.s3.amazonaws.com/questions/1890011_1909650_ans_86e4b88529e541639e84039753e5b955.png)
0 Response to "42 lewis dot diagram of ammonia"
Post a Comment