42 sf6 molecular orbital diagram
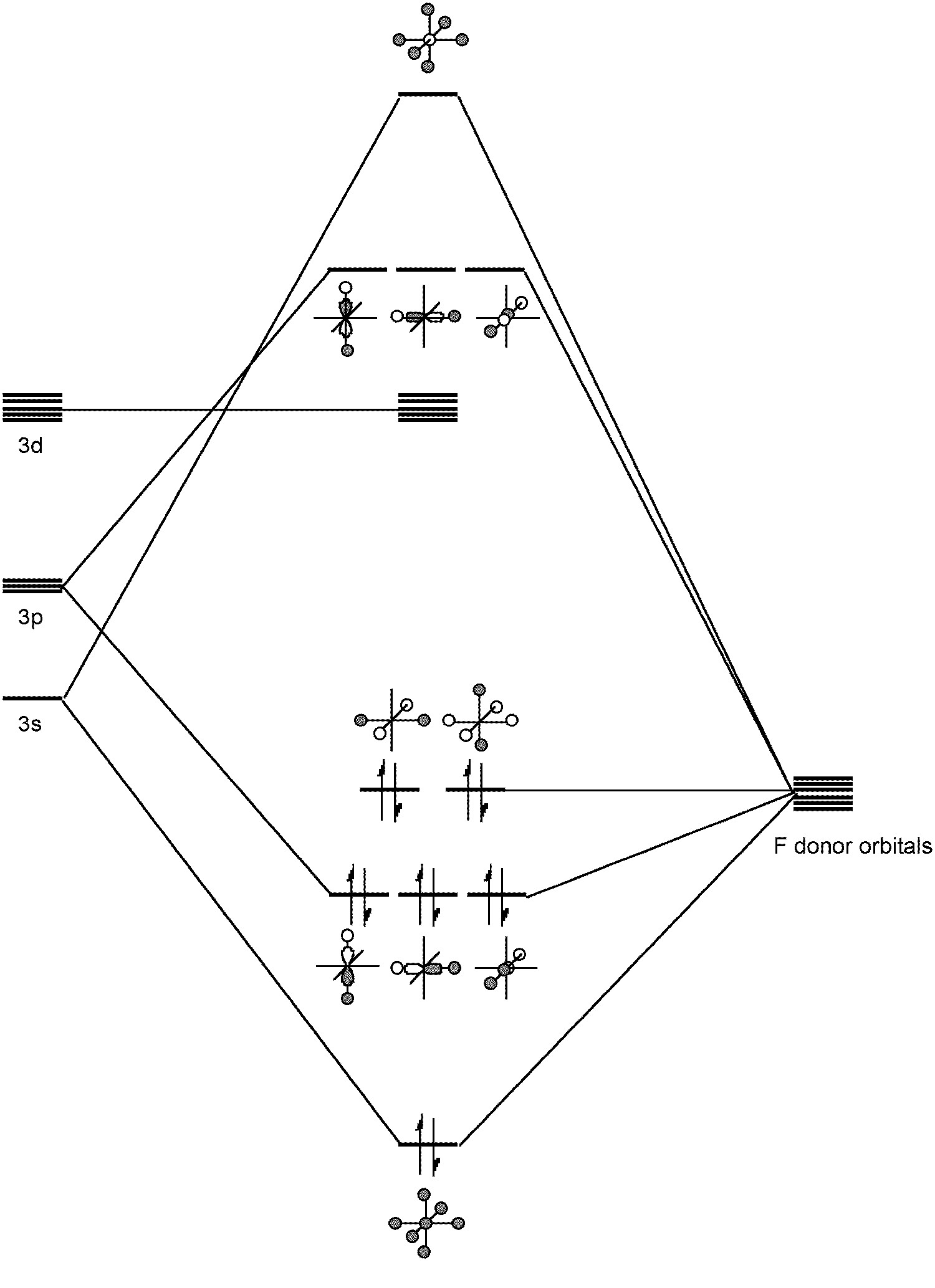
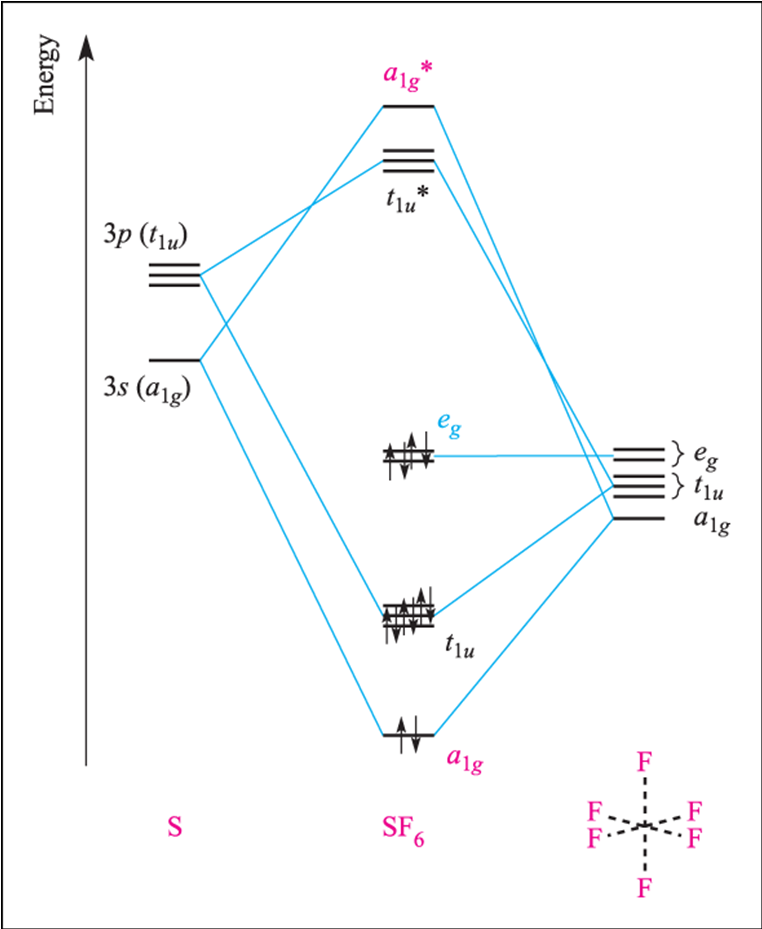
SF4 Lewis Structure, Molecular Geometry, Hybridization ... SF4 or sulfur tetrafluoride is a compound that has a distinct odor of sulfur or rotten eggs. This compound is generally identified as being a colorless gas. The molecular weight of this compound is calculated to be 108.6 g/mol. SF4's boiling and melting points are -38 degrees Celcius and -121 degrees Celcius respectively. Solved Draw the molecular orbital diagram for octahedral ... Draw the molecular orbital diagram for octahedral SF6. Treat each F ligand as though it is spherically symmetrical (like an H atom) to construct your SALCs. Mix these SALCs (which can start at -20 eV) with the valences and p orbitals of sulfur (which have energies of -20.7 and -11.6 eV, respectively).
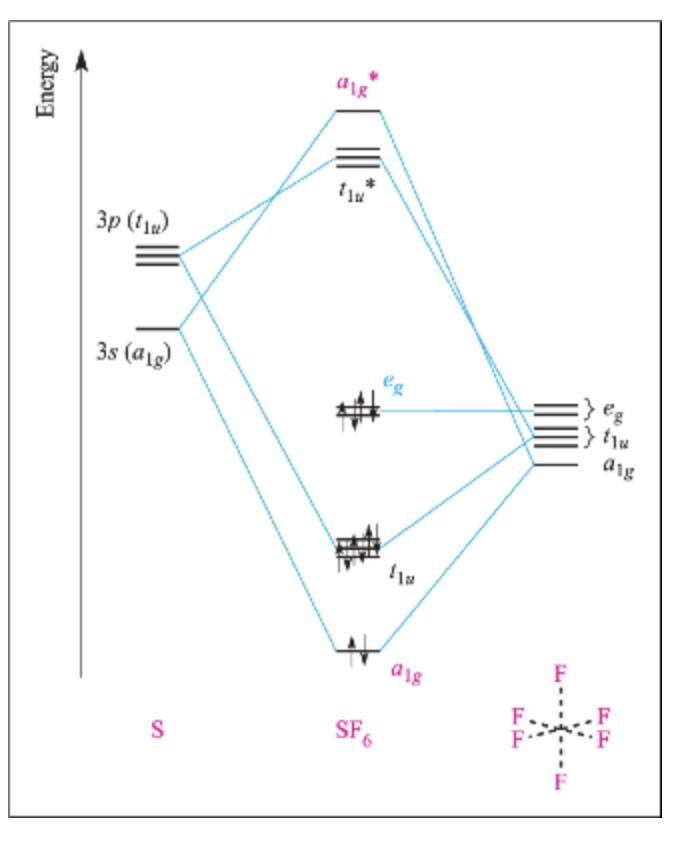
Molecular orbital theory(mot) of SF6/CO2/I3-/B2H6 Molecular Orbitals in SF6 The Lewis structure of SF6 describes six pairs of electrons as bond pairs, despite the availability of only four valence orbitals on sulphur. The valence bond description of this hypervalent complex must therefore invoke d- orbitals.

Sf6 molecular orbital diagram
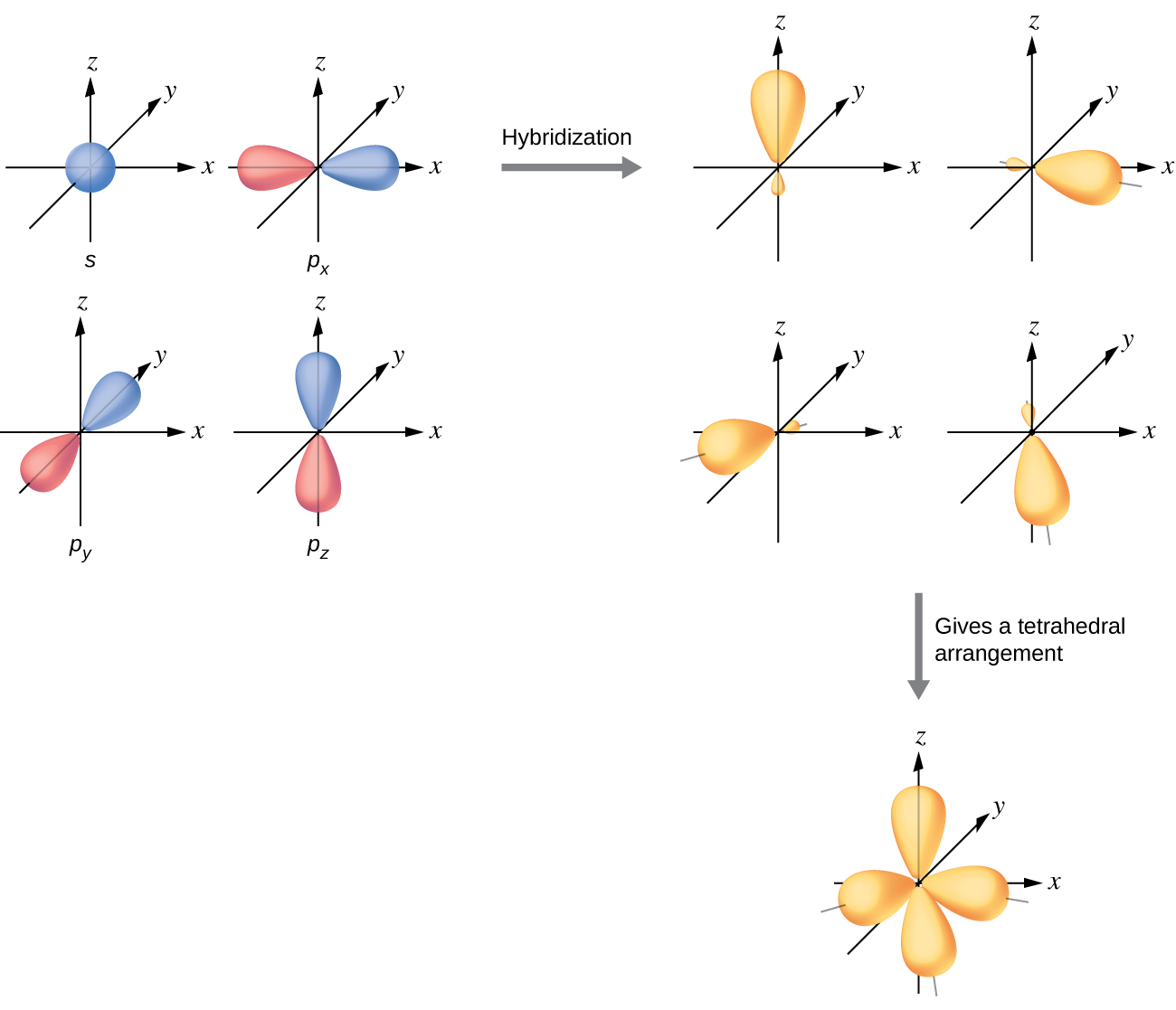
What is the structure of SF6? - Quora The geometry of SF6 molecule can be explained on the basis of sp3d2 Hybridization. To account for the hexavalency in SF6 one electron each from 3s and 3p orbitals is promoted to 3d orbitals. These six orbitals get hybridised to form six sp3d2 hybrid orbitals. Each of these sp3d2 hybrid orbitals overlaps with 2p orbital of fluorine to form S-F bond. Molecular orbitals in SF6 : chemistry - reddit SF6 is octahedrally symmetric. To visualize the symmetry operations you can carry out on this molecule, go to this site, select "Oh" under the "point group type" menu, and then select sulfur hexafluoride. Notice that there are no unique fluorines. Each can be interchanged with another by those symmetry operations. Answered: In the construction of the molecular… | bartleby In the construction of the molecular orbital diagram of a polyatomic molecule (SF6), 12 valence electrons are used to occupy 10 MO; account for the origin of the 12 electrons and the 10 MOs Question thumb_up 100%
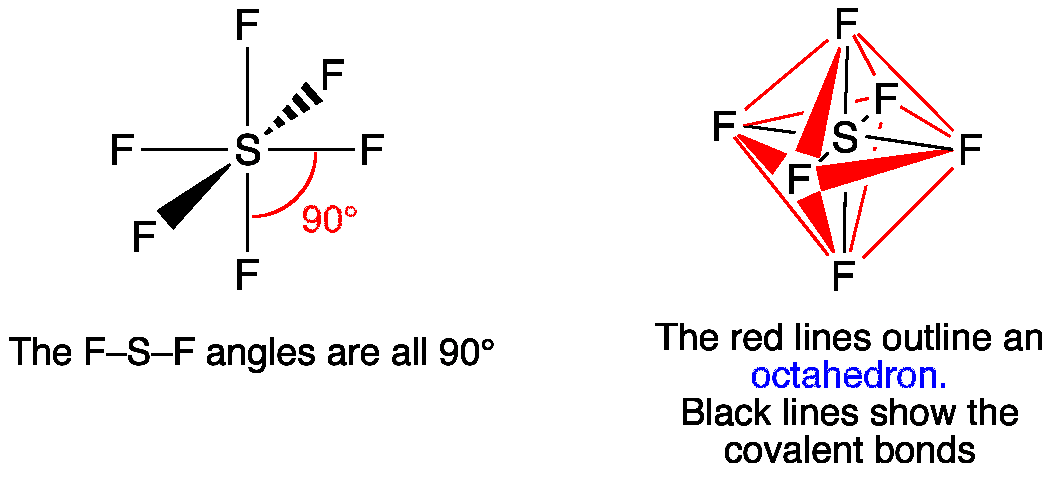
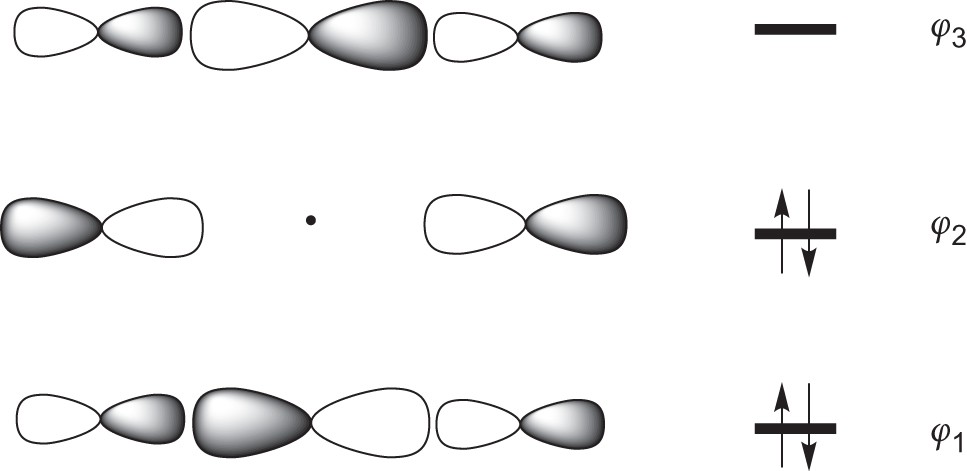
Sf6 molecular orbital diagram. Octahedral Sulfur Hexafluoride - SF6 - Oh Octahedral Sulfur Hexafluoride - SF 6 - O h CONTROLS Click the buttons labelled with Symmetry Operations below to view in 3D O h point group contains 3 C 4, 4 C 3, 9 C 2, 4 S 6, 3 S 4, 3 σ h, 6 σ d and a centre of inversion Inversion operation is a reflection through the centre of the molecule. Orbitals And Molecular Representation The bond angle of F-S-F is 90 degrees. SF6 Molecular GeometryBonding molecular orbitals are lower in energy and hence, more stable than individual atomic orbitals whereas antibonding molecular orbitals will be higher in energy than individual atomic orbitals and hence, less stable. We have SF6 MO diagram - YouTube About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ... Explaining the bonding of SF6 if hybridisation of d ... One of the ways it can be explained is that there are multiple resonance hybrids with 4 S-F bonds and 2 F- S+ ionic bonds - ie this sort of item. Another one is the 3 centre 4 electron bond which has 3 molecular orbitals that look like this. It's a much better way of looking at it and doesn't involve using the d orbitals, but it also relies on ...
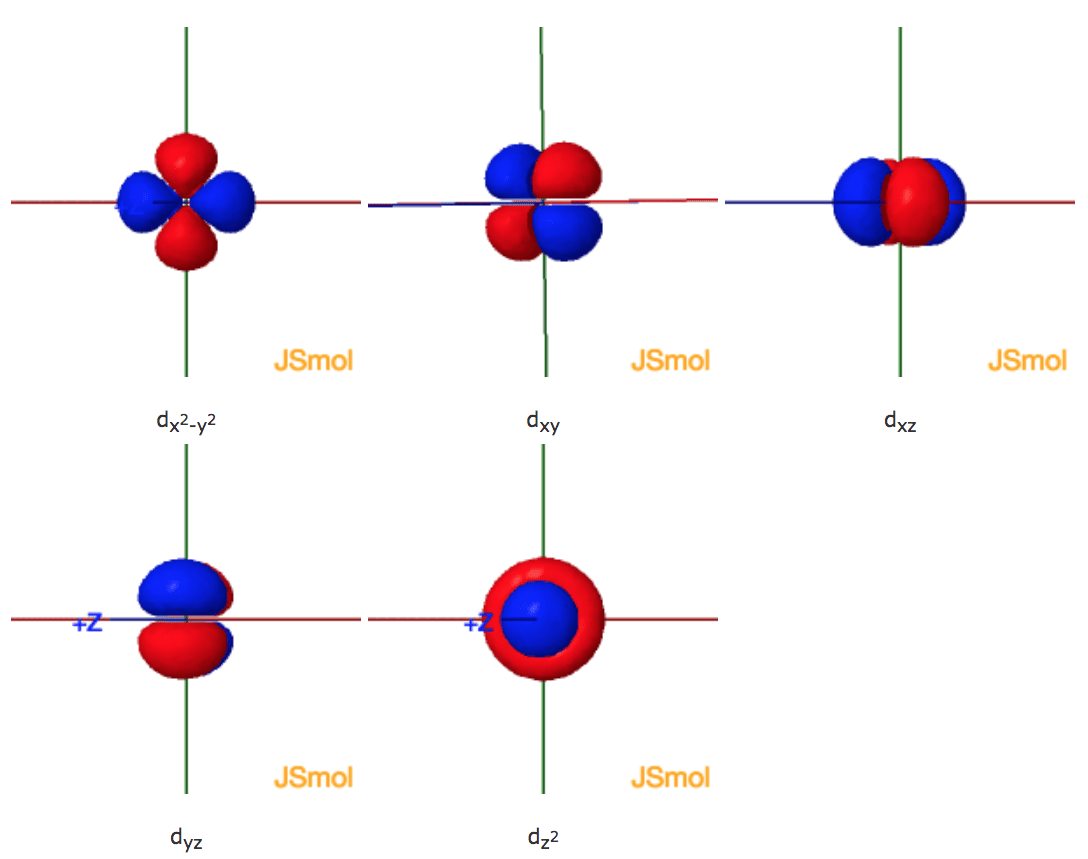
On the role of d orbitals in sulfur hexafluoride | Journal ... Molecular Structure and Pseudorotation of 1,1-Dichlorocyclopentane As Determined by Gas-Phase Electron Diffraction and ab Initio Calculations: A Large Amplitude Treatment. The Journal of Physical Chemistry A 2004, 108 (21) , 4658-4673. Solved In the Molecular Orbital diagram of SF6 , | Chegg.com In the Molecular Orbital diagram of SF6 , these sulfur orbitals are non-bonding: px , py , pz dxy , dxz , dyz dx2-y2 , dz2 S Can you explain your answer? Expert Answer Previous question Next question inorganic chemistry - Why do compounds like SF6 and SF4 ... Shown in Figure 12 is the orbital occupation of the sulfur center, 3 s X 1 3 p X 2.1 3 d X 0.19 5 p X 0.03 4 f X 0.01. The minimal occupation of d-type orbitals eliminates the possibility of s p 3 d 2 hybridization. If not via d-orbital bonding, how does one then describe the structure of S F X 6? I'll present an LCAO-MO answer. Atomic orbitals involved in hybridization of SF6 molecule: Click here👆to get an answer to your question ️ Atomic orbitals involved in hybridization of SF6 molecule: Solve Study Textbooks. Join / Login >> Class 11 >> Chemistry >> Chemical Bonding and Molecular Structure >> Hybridization >> Atomic orbitals involved in hybridizatio. Question .
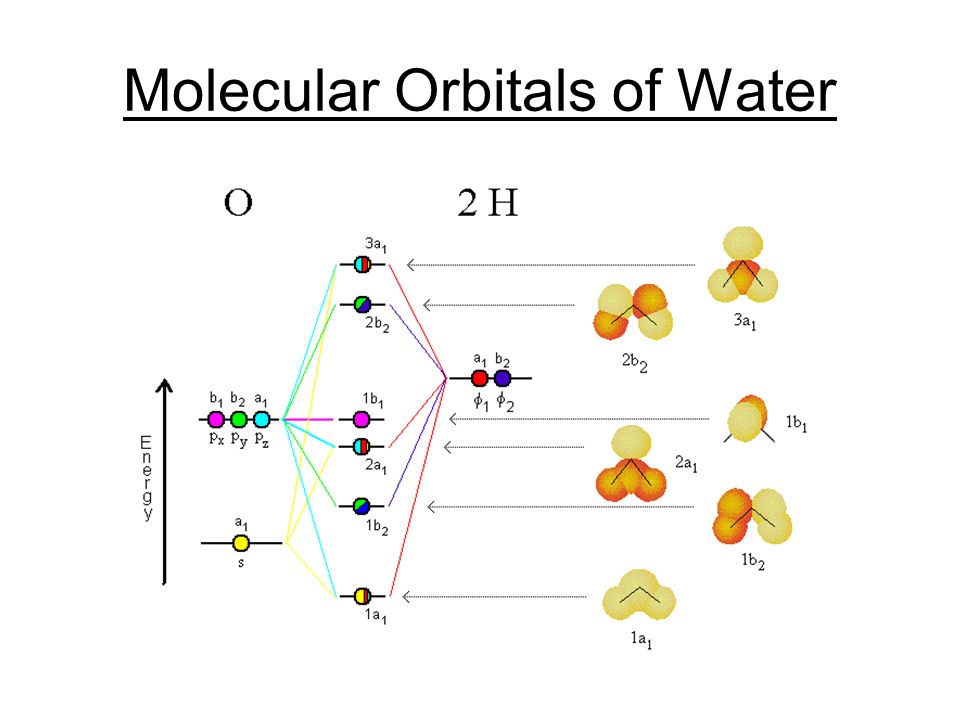
PDF Hybridisation of s in sf6 fluorine atoms form bonds. These six orbitals are in six directions of octahedron shape. Then, sulfur hexafluoride has the SP3D2 hybridization. Angle SF6 Sulfur Bond shares its valence electrons with 6 fluorine atoms, we can see that all six sulfur atom electrons are shared form bonds. The bond angle of F-S-F is 90 degrees. Molecular Geometry SF6 SF6 Lewis Structure, Molecular Geometry, Hybridization ... SF6 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram SF6 or sulfur hexafluoride is an inorganic and one of the most stable gases that are known in chemistry. This gas has more density than air. The general identification of the gas can't be done because it is odorless and colorless in nature. PDF Construction of Molecular Orbitals The most common ... The are three features of the MOs that are worth noting: (1) Starting with Natomic orbitals, we can construct Nmolecular orbitals. No more and no less. (2) The MOs are mutually orthogonaland normalized. This means that the net overlap between any two MOs is zero and the net overlap of any MO with itself = 1. PDF Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules An atomic orbital is located on a single atom. When two (or more) atomic orbitals overlap to make a bond we can change our perspective to include all of the bonded atoms and their overlapping orbitals. Since more than one atom is involved, we refer to these orbitals as molecular orbitals.
Hypervalent molecule - Wikipedia An LCAO in, for example, sulfur hexafluoride, taking a basis set of the one sulfur 3s-orbital, the three sulfur 3p-orbitals, and six octahedral geometry symmetry-adapted linear combinations (SALCs) of fluorine orbitals, a total of ten molecular orbitals are obtained (four fully occupied bonding MOs of the lowest energy, two fully occupied ...
Sulfur-Hexafluoride (SF6) - ElectricalSchool.org Sulfur-Hexafluoride (SF6) Definition: A very dense, inert, non-conducting gas used inside high voltage equipment to insulate conducting components from surfaces at ground potential. It also is used as an interrupting medium in high voltage circuit breakers. Sulfur hexafluoride - Wikipedia
Molecular Orbital diagram of SF6 molecule - YouTube Ligand group orbitals of SF6 and nature of MOs, Electronic structure of SF6
(Get Answer) - For the molecule SF6 a. Draw the molecule ... For the molecule SF6 a. Draw the molecule, indicating the 3-center-4-electron bonding. b. Provide the irreducible representations of the s-bonds of the 6 F atoms. c. Draw the molecular orbital diagram, labeling each molecular orbital by its symmetry (e.g. a1g). d. How many bonding, non-bonding, and...
SF6 Molecular Geometry, Lewis Structure, Shape, and Polarity The electronic configuration of SF6 in its ground state is 3s23p4. But when it shares electrons and is in the excited state the electron pairs in both 3s and 3p orbitals get unpaired. These electrons move to fill the higher vacant 3d orbitals. As a result, six hybrid orbitals are formed ( one of 3s, three of 3p, and two 3d).
Bef2 Molecular Orbital Diagram - Wiring Diagrams A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.Chemistry: The Central Science, Chapter 9, Section 5Chapter 9 - Molecular Geometry.
OneClass: Construct a Molecular Orbital Diagram showing ... (iv) Construct a molecular orbital energy diagram for SF6 (v) Determine the bond order for S-F in SF6. (vi) Figure 5.27 in your text gives the group orbitals for 6F2p. Sketch any possible bonding combinations between the non-bonding group orbitals and S 3d orbitals.
Question regarding "Molecular Bond of SF6" - CHEMISTRY ... Question regarding "Molecular Bond of SF6". Postby Yinhan_Liu_1D » Sat Nov 05, 2016 5:07 pm. In the textbook p140, it mentions that "the four valence orbitals provided by the sulfur atom and the six orbitals of the fluorine atoms that point toward the sulfur atom, a total of 10 atomic orbitals..." 1) Why does sulfur atom has FOUR valence orbitals?
Hybridization of SF6: Hybridization of S in Sulfur ... During the formation of SF 6, the sulphur atom which is the central atom in its ground state will have 3s 2 3p 4 configuration. In the excited state, the electron pairs in 3s and 3px orbitals get unpaired and one out of each pair is promoted to vacant 3d z2 and 3d x2-y2 orbitals.
PDF Michaelmas Term - Second Year 2019 - University of Oxford 3. The nature of molecular orbitals 4. The linear combination of atomic orbitals (LCAO) approach to molecular orbitals Diatomic molecules: H2 +, H 2 and AH 5. The wave functions for H2 + and H 2 using an LCAO approach 6. MO schemes for AH molecules (A = second period atom, Li to F) Symmetry and molecular orbital diagrams for the first row ...
Answered: In the construction of the molecular… | bartleby In the construction of the molecular orbital diagram of a polyatomic molecule (SF6), 12 valence electrons are used to occupy 10 MO; account for the origin of the 12 electrons and the 10 MOs Question thumb_up 100%
Molecular orbitals in SF6 : chemistry - reddit SF6 is octahedrally symmetric. To visualize the symmetry operations you can carry out on this molecule, go to this site, select "Oh" under the "point group type" menu, and then select sulfur hexafluoride. Notice that there are no unique fluorines. Each can be interchanged with another by those symmetry operations.
What is the structure of SF6? - Quora The geometry of SF6 molecule can be explained on the basis of sp3d2 Hybridization. To account for the hexavalency in SF6 one electron each from 3s and 3p orbitals is promoted to 3d orbitals. These six orbitals get hybridised to form six sp3d2 hybrid orbitals. Each of these sp3d2 hybrid orbitals overlaps with 2p orbital of fluorine to form S-F bond.













0 Response to "42 sf6 molecular orbital diagram"
Post a Comment