37 benzene molecular orbital diagram
15:39Chad provides a lesson on the Pi Molecular Orbitals of Benzene and ... MO diagrams of aromatic and ...1 Mar 2021 · Uploaded by Chad's Prep 6:56AboutPressCopyrightContact usCreatorsAdvertiseDevelopersTermsPrivacyPolicy & SafetyHow YouTube ...12 Jul 2020 · Uploaded by ChemistryTuition
This section provides the lecture notes from the course and information on lecture topics.

Benzene molecular orbital diagram
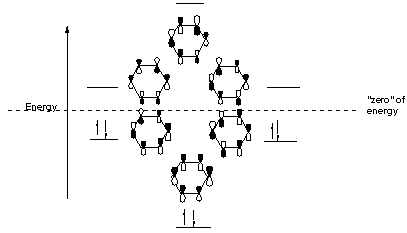
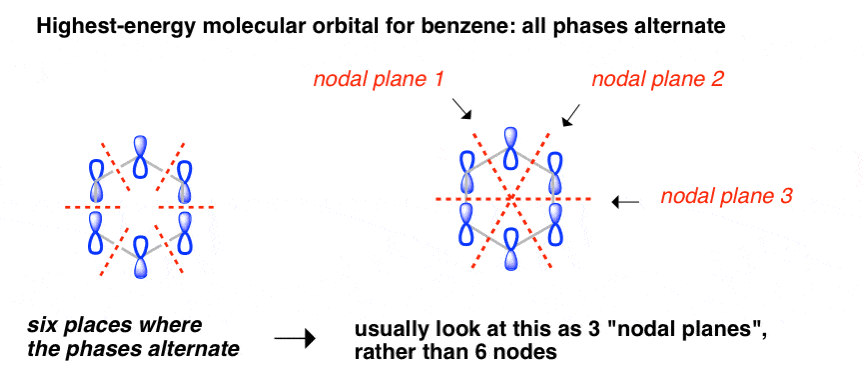
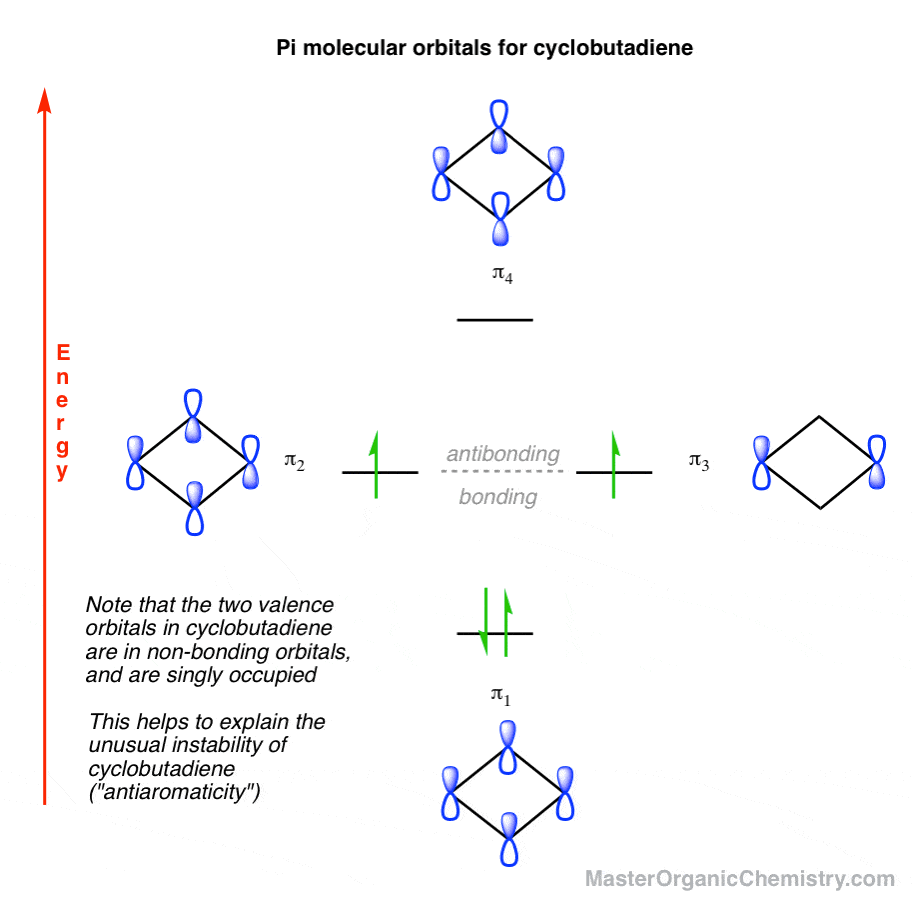
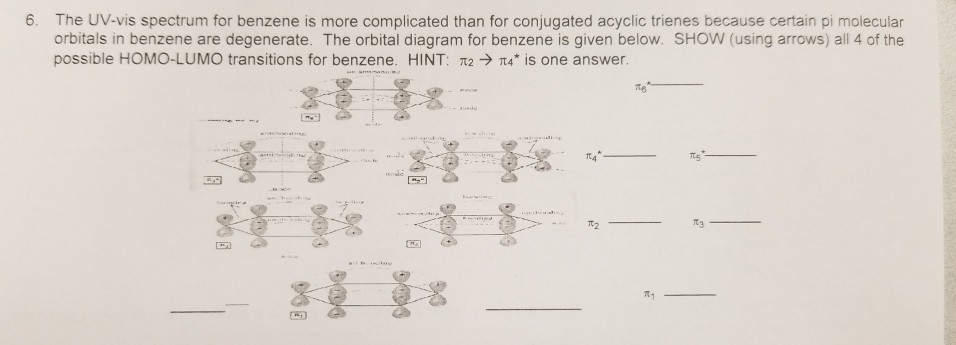
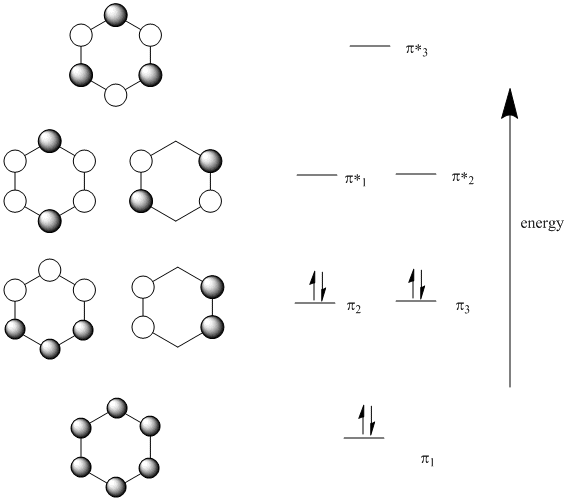
Therefore, according to Hund's ... orbitals - are fully occupied, while the three antibonding molecular orbitals remain empty. The resulting electron configuration is considerably low in energy. Benzene's high stability can partially be explained by its molecular orbitals ... 5 May 2017 — Start with the π6 molecular orbital of benzene, which has six sites where the phases change. Flipping the phases of any one of those p orbitals ... July 14, 2020 - The pi1 molecular obrital of benzene (Left) has 6 stabilizing bonding interaction ... 1) The molecule, pyridine, is planar with bond angles of 120o. Pyridine has many other characteristics similar to benzene. Draw a diagram showing the p orbitals in pyridine and use it to explain its similarity ...
Benzene molecular orbital diagram. Home of Vanderbilt's intellectual omnivores · All programs are Ph.D. programs unless otherwise noted Donate here: http://www.aklectures.com/donate.php Website video link: http://www.aklectures.com/lecture/molecular-orbitals-of-benzene Facebook link: https://... Sie scheinen keine Seiten mit Frames betrachten zu können - klicken Sie hier zur Umgehung des Frames · Your browser seems not to support frames - click here to circumvent the frame Enjoy the videos and music you love, upload original content, and share it all with friends, family, and the world on YouTube.
This video illustrates deep molecular orbital analyses of benzene and hexatriene. It reiterates many of the concepts we have discussed already in terms of th... Clearly it takes something more to be aromatic, and this can best be explained with molecular orbital theory. Let’s look at an energy diagram of the pi molecular orbitals in benzene. October 5, 2020 - Read Or Download Orbital Diagram For FREE Of Benzene at 600AFUSE7542.TRATTORIAFERI.IT The orbital structure of benzene: All the carbon atoms in benzene are sp2 hybridised. The three sp2 hybrid orbitals are lying in one plane and oriented at an angle of 120°. The fourth unhybridized p-orbital having two lobes is lying perpendicular to the plane of the hybrid orbital.
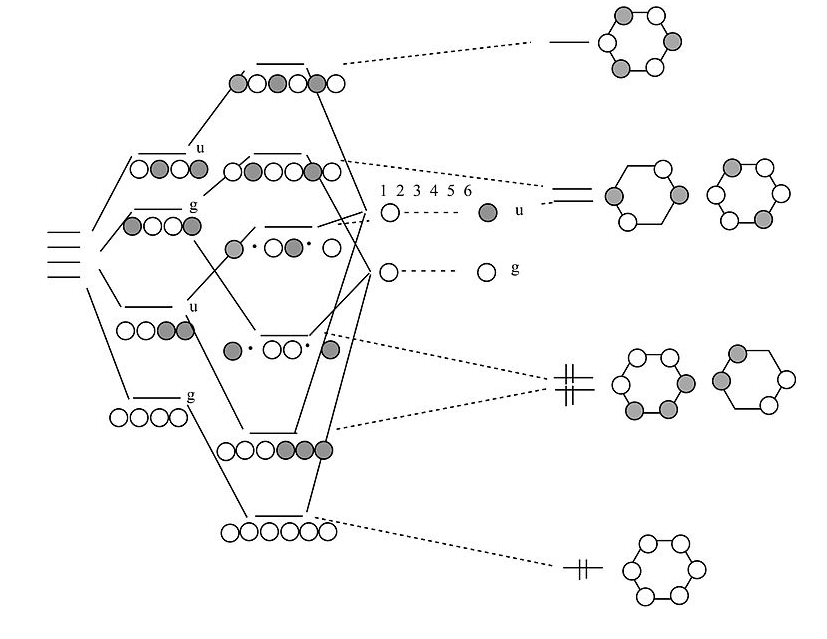
Resonance structures of benzene and the resonance hybrid. Survey of the molecular orbitals of benzene. Degeneracy. Patterns in orbital energy and number of n... July 11, 2020 - Clearly it takes something more to be aromatic, and this can best be explained with molecular orbital theory. Let’s look at an energy diagram of the pi molecular orbitals in benzene. C-C length in alkane = 1.54oA · C=C length in alkene = 1.34oA March 16, 2020 - Interactive 3D chemistry animations of reaction mechanisms and 3D models of chemical structures for students studying University courses and advanced school chemistry hosted by University of Liverpool
14:21Benzene#engineeringchemistry#chemistry#chem1#Lastmomenttuitions #lmt In This Video ... pi Molecular ...18 Nov 2019 · Uploaded by Last moment tuitions
You need to enable JavaScript to run this app
An explanation of the bonding in benzene, including the delocalisation of the pi electrons
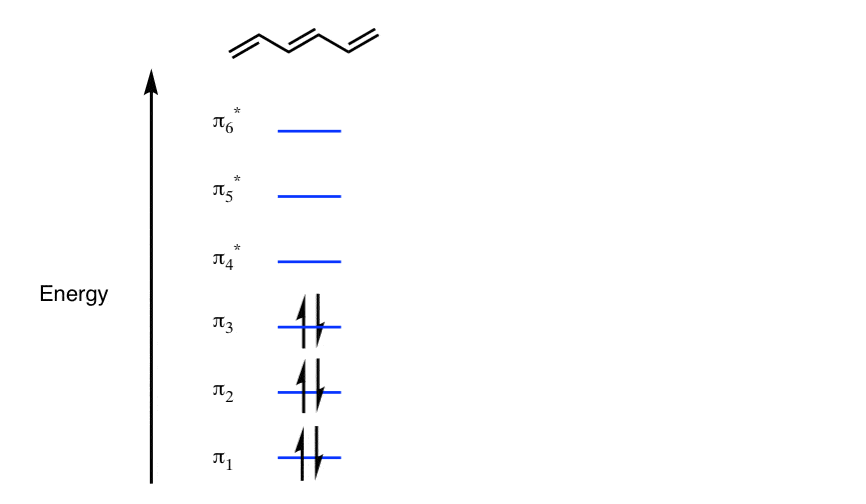
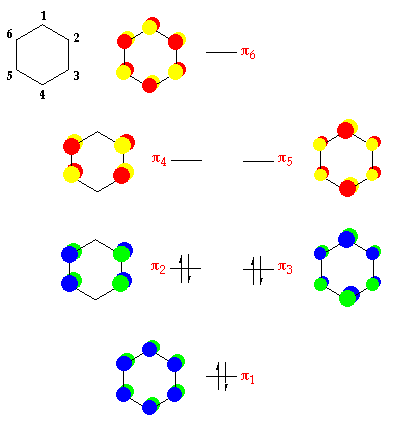
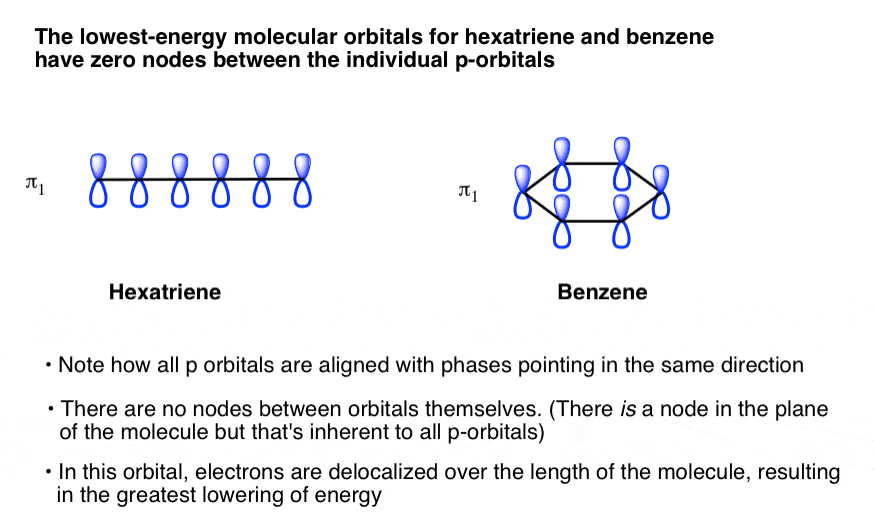
Now we will look at the π molecular orbitals for benzene. With 6 C atoms contributing to the π system, we need to create 6 molecular orbitals. These are shown below. The most stable orbital, ▁, is the all in-phase combination. We then have two orbitals, ▂ and ▃, that ...
Explanation of bond order of benzene using molecular orbital theory I am a private chemistry tutor offering tuition online to students around the globe. Plea...
July 14, 2020 - The pi1 molecular obrital of benzene (Left) has 6 stabilizing bonding interaction ... 1) The molecule, pyridine, is planar with bond angles of 120o. Pyridine has many other characteristics similar to benzene. Draw a diagram showing the p orbitals in pyridine and use it to explain its similarity ...
5 May 2017 — Start with the π6 molecular orbital of benzene, which has six sites where the phases change. Flipping the phases of any one of those p orbitals ...
Therefore, according to Hund's ... orbitals - are fully occupied, while the three antibonding molecular orbitals remain empty. The resulting electron configuration is considerably low in energy. Benzene's high stability can partially be explained by its molecular orbitals ...

Lewis Structure Atomic Orbital Molecular Orbital Diagram Orbital Hybridisation Molecular Chain Angle Text Chemistry Png Pngwing

Molecular Orbital Treatment Of Benzene Bond Lingth Analysis In Benzene Modern Representation Stability Of Benzene Ring
Chapter 1 Molecular Orbital Concepts A Concepts Of Mo Theory 1 Strong Covalent Bonds Consider The Pi Bond Of Ethene In Simple Molecular Orbital Terms The Qualitative Results Would Be The Same For Any Pi Or Sigma Bond Q The Overlap Of The Two
Why Does The Reaction Take Place On The Central Ring Of Anthracene In A Diels Alder Reaction Socratic

Pdf Minimum Change Of Shapes Of Molecular Orbitals In The Elementary Chemical Reactions And A New Perspective Of Quantum Chemistry Semantic Scholar

Discuss The Molecular Orbital Structure Of Benzene Delocalisation Of Electrons From Chemistry Hydrocarbons Class 11 Haryana Board English Medium

How Amino And Nitro Substituents Direct Electrophilic Aromatic Substitution In Benzene An Explanation With Kohn Sham Molecular Orbital Theory And Voronoi Deformation Density Analysis Physical Chemistry Chemical Physics Rsc Publishing















0 Response to "37 benzene molecular orbital diagram"
Post a Comment