38 lewis diagram for nh3
Lewis Dot Diagram Of Nh3 The Lewis structure of ammonia NH3 would be three hydrogen atoms bonded to a nitrogen atom in the middle with a lone pair of electrons. Ammonia NH3 or H3N CID 222 - structure chemical names physical and chemical properties classification patents literature biological activities safety. It also is a good example of a ... Chemical Formula Total Number of Valence Electrons Lewis Dot Structure CH4 NH3 CF4 CO2 BF3 C4H6 H2O H2 Cl2 PF3 HF HCl N2 C2H4 Title. Electron Dot Structure or Lewis Dot Diagram Gilbert Lewis A notation showing the valence electrons surrounding the atomic symbol. View Lewis Structure worksheet1doc from CHEM 525-01 at Bishop Fenwick High School.
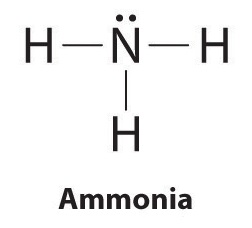
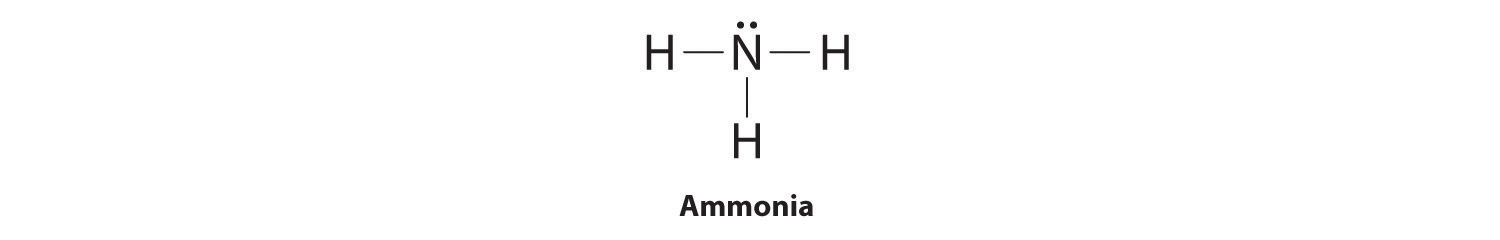
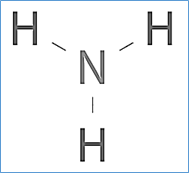
The Lewis structure of ammonia, NH3 , would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons on top of the atom. This is the reason why ammonia acts as a Lewis base, as it can donate those electrons. Should BH3 and NH3 have the same shape? NH3 has 3 bondpairs and 1 lonepair. whereas BH3 has 3 ...
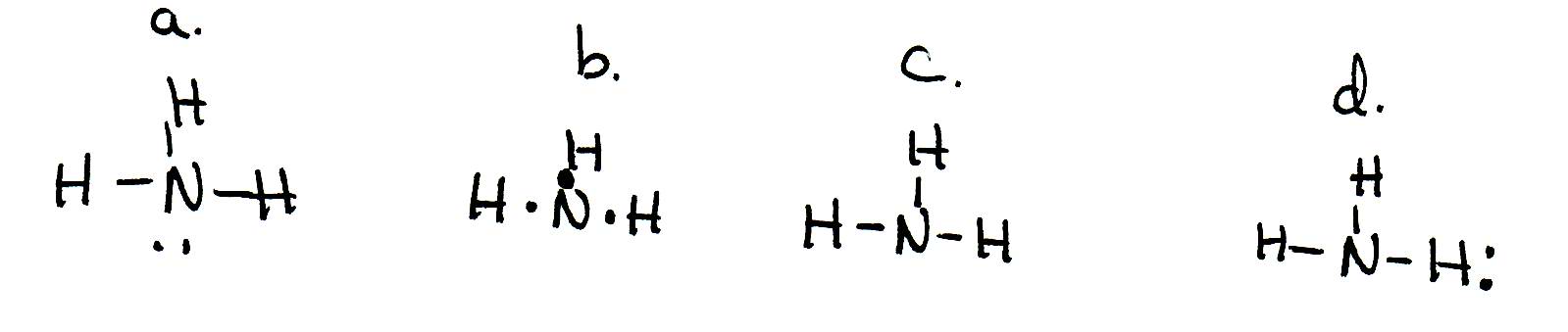
Lewis diagram for nh3
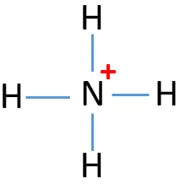
The lone pair in NH3 is given to the H+ ion to do a brand-new N-H bond. Now, over there are 4 N-H bonds around nitrogen atom. See the reaction that ammonia and also HCl. Lewis framework of NH4+ has a +1 charge on nitrogen atom. Therefore is it a secure structure? First, we should know, atoms in a lewis structure can contain charges. Is Ammonia or NH3 Lewis Acid Or A Base? NH3, likewise identified as Ammonia, is a pungent-smelling gas substance that comprises one atom of Nitrogen and three hydrogen atoms. Ammonia has a low boiling temperature level at -33 degrees Celsius as well as is lighter than air. Often students guess about whether NH3 is an acid or Base. Ammonia is a compound of nitrogen and hydrogen with the formula NH 3.A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. It is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to 45 percent of the world ...
Lewis diagram for nh3. Intermolecular Forces ... Lewis Diagram: Ammonia NH3 ... Lewis Diagrams and Formal Charges: NITRITE Ion NO2 Boron is the least electronegative atom in the BF3 Lewis structure and therefore goes at the center of the structure. What is the structure of bf3 and NH3? NH3 has three bonds and one lone pair of electrons and is trigonal pyramidal. BF3 is a molecule in the trigonal planar shape. Nh3 lewis structure 3d model . Drawing the lewis structure for nh 3 ammmonia ammonia nh 3 is a commonly tested lewis structure due to it s widespread use in agriculture as a fertilizer. You do not need the phet simulations. The lewis dot structure for nh3 ammonia is shown above. Nh3 lewis structure 3d model . Compare h2o nh3 and ch2cl2. Drawing the Lewis structure for C2H4 (named ethene) requires the use of a double bond. In a double bond two pairs of valence electrons are shared (for a total of four valence electrons). Does C2H4 have lone pairs? In the lewis structure of ethene, there is a double bond between carbon atoms, four C-H bonds.
NH3 Lewis Structure The Lewis structure of a molecule helps understand the electron geometry, molecular geometry, polarity and other such properties with ease. It is a pictorial representation of the arrangement of valence electrons around the individual atoms in the molecule. I am in college General Chemistry, and we did [this lab](https://www.dropbox.com/s/1c0n58jrrps5uzp/Lab%207%20Magnetic%20Nanoparticles.pdf?dl=0) despite not covering the material in lecture, so I really have no idea how to go about it. Here are the questions I was given: Write the equation and net ionic equation for the observed reactions in Part 1. 2. Define the limiting agent in the reaction. And in what excess of ammonia was the magnetite (Fe3O4) synthesized, i.e. 2-fold, 5-fold, 10-fold... ... Diagram Under Dash , Jumper Cable Hookup Diagram , Sensory Neuron Diagram , Train Horn Wiring Diagram Without Relay , Trailer Light Plug Diagram , ... What is the Lewis structure of NH3? Why ammonia acts as a Lewis base because it can donate those electrons. The (NH3) molecule features a trigonal pyramidal shape as predicted by the valence shell electron pair repulsion theory (VSEPR theory) with an experimentally determined bond angle of 106.7°.
10+ Nh3 Lewis Structure. This is the reason why ammonia acts as a lewis base, as it can donate those electrons. (1) you have two chlorine atoms. Bonding and structure in covalent compounds. Electron dot structures or lewis dot formula can be drawn if the molecular formula of the compound is known. Source: i.ytimg.com. ... File Refrigerationts Itg Wikimedia Mons – Refrigeration Cycle Pv Diagram ... Ford Ranger Front Suspension Diagram ... Lewis Dot Diagram For Nh3 NH3 Lewis Structure, Molecular Geometry, Hybridization, Bond Angle & Shape. Ammonia is a colorless compound, used in making fertilizers. It is a stable hydride formed of one nitrogen and three hydrogen atoms. The molecule has a pungent smell. It can form an NH4+ ion by accepting a proton. Ammonia (NH3) is a colourless, pungent gas and is consisted of of nitrogen and hydrogen. In the NH3 Lewis structure, three hydrogen atoms are bound come a nitrogen atom. NH3 molecule geometry is of pyramidal form with triangle pyramidal geometry.
organic-chemistry , lewis-structures-and-bonding , lewis-dot-diagram , nh3-lewis-structure , nh3 , nh3-structure ... Lewis Structure Lewis Structure ...
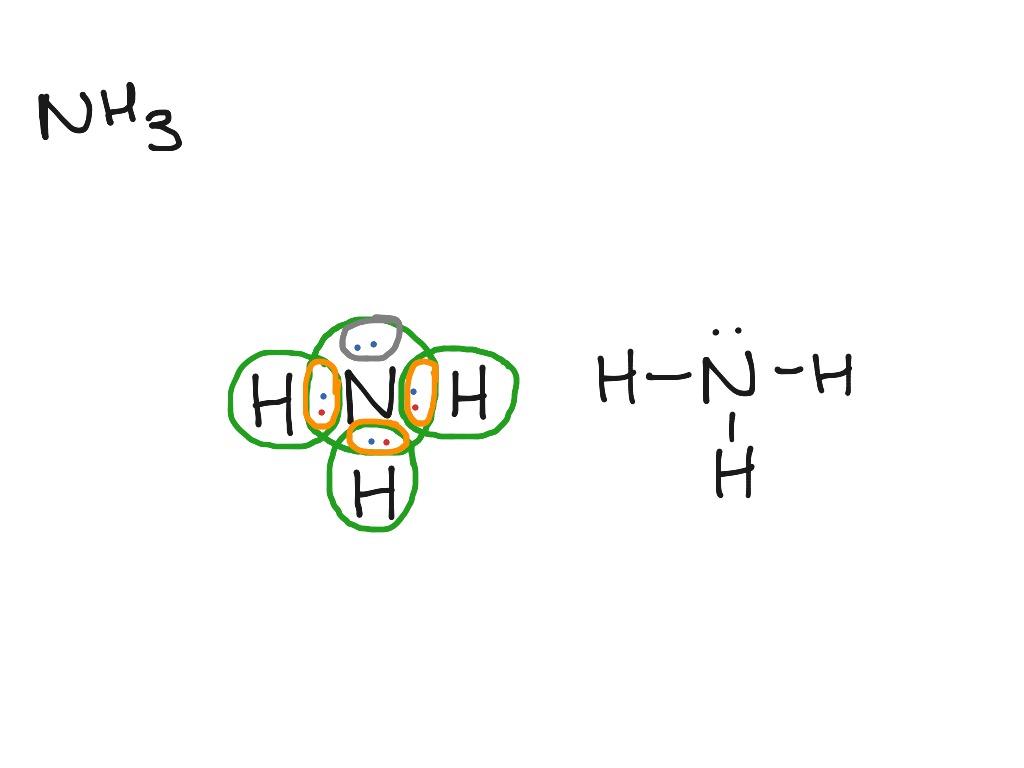
NH3 Lewis Structure. The Lewis framework of a molecule helps recognize the electron geometry, molecular geometry, polarity and other such properties with ease. It is a photographic representation the the plan of valence electrons about the individual atoms in the molecule.
The bond angle of N-H in the NH3 molecule is around 106.7 degrees. The hybridization of the ammonia molecule is sp3. Below is the lewis structure of the Ammonia molecule for better understanding. You must also go through the article written on NH3 Lewis Structure, Molecular Geometry, and Hybridization. Factors that determine the polarity
The Lewis Dot Structure for NH3 ... The Lewis Dot Structure for NH3 (Ammonia) is shown above. ... formed between two Hs because the relatively small ...
The NH 3 Lewis structure has the typical case of nitrogen N in the center with 3 bonds to 3 other atoms. Its not particularly difficult but is an important structure. The Lewis structure for NH3 isThe Lewis dot structure for NH3 starts with an N atom connected on three sides with a dash each to an H atom. By crator-avatar Jeff Bradbury 2.

Draw The Lewis Structure For Nh3 How Many Single Double And Triple Covalent Bonds Are Present In This Molecule What Is Its Molecular Geometry Is It Polar Or Nonpolar Study Com
# Why is A Level Chemistry So Hard? If you are taking A Level Chemistry, you will probably agree, like most students, that A Level H2 Chemistry is difficult and you have good reasons to do so. The concepts are complex and involve much memory work. There is a steep increase in the learning curve. This is only natural. Having now graduated from the top 20% of the O Level cohort, the syllabus is now made much tougher to further differentiate among all of you. # Much Memory Work is Required C...
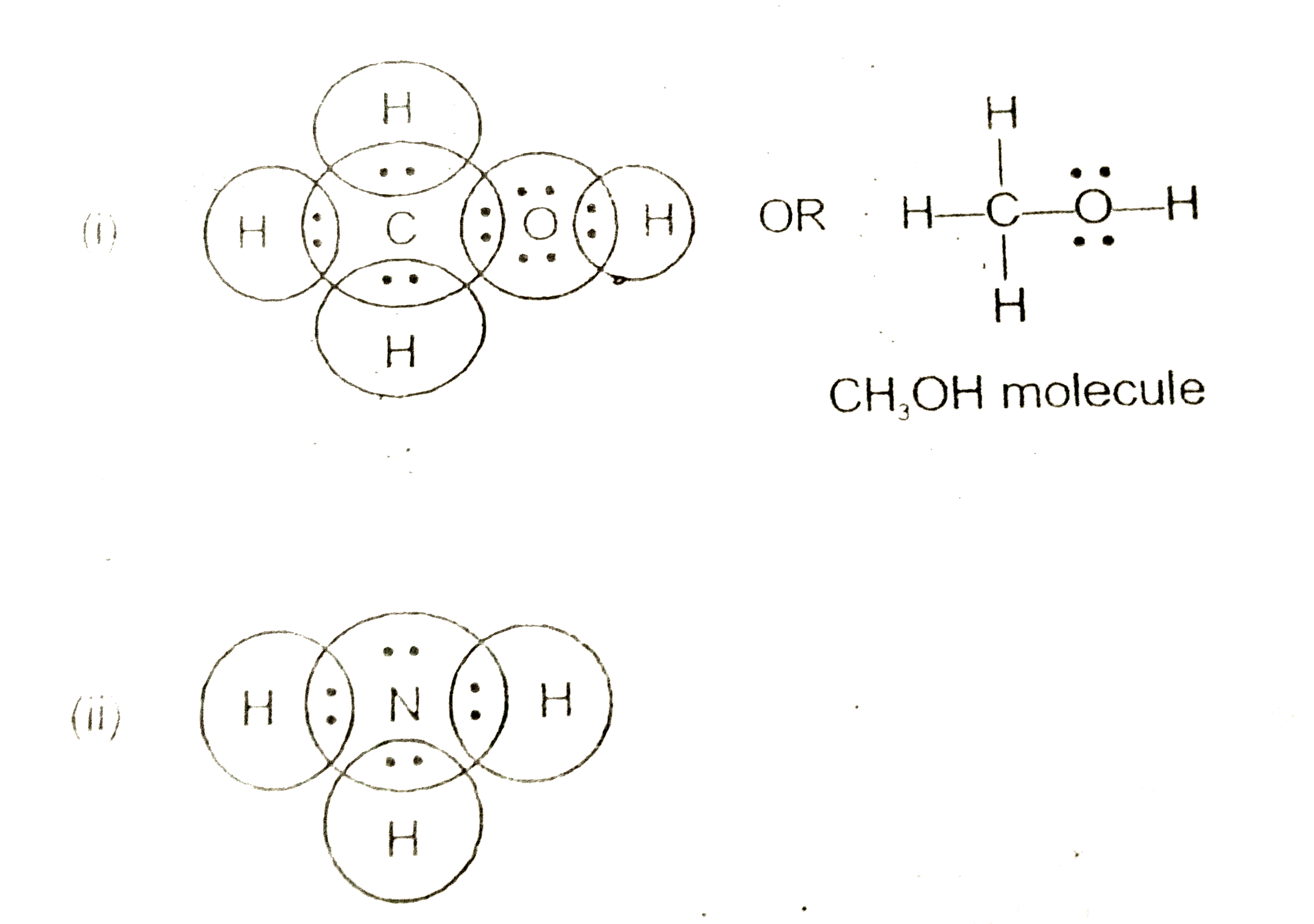
Draw The Lewis Dot Structure Of The Following Molecules I Methyl Alcohol Ch 3 Oh Ii Ammonia Molecule Nh 3
... Diagram , Glucose Diagram , Kenworth Door Parts Diagram , 2003 Ford Explorer Fuse Box Diagram , International Space Station Diagram , 7018b Wiring ...
... Diagram , Jvc Car Stereo Wiring Diagram Color , Milk Ducts Diagram , Car Front End Parts Diagram , Blank Venn Diagram Template , Dolphin Diagram , ...
NH3 Lewis Structure, Geometry, and Hybridization. Ammonia is the simplest binary hydride made up of nitrogen and hydrogen denoted by its chemical formulae as NH3. It is a stable pnictogen hydride where all the atoms are covalently bonded to achieve a reactive state. Ammonia is lighter than the air, colorless, and pungent in smell.
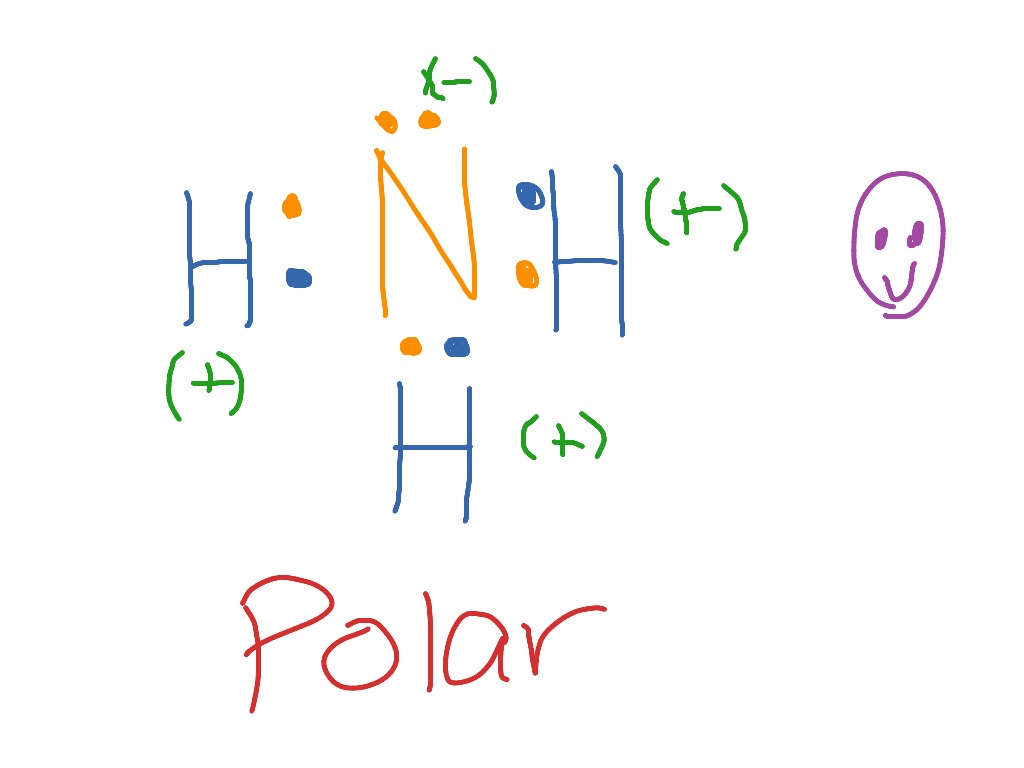
Yes, hydrogen bonding occur between nh3 molecules because if you look at the structure of nh3, hydrogen are directly attached with high electronegative atom such as nitrogen (N). and we know that, if any molecules bound to highly electronegative elements then they generate hydrogen bonds between two molecules such as, N-H, H-O, and H-F then it ...
Nh3 Lewis Structure Dipole. October 08, 2021. Ljmong8psaejbm. Nh3 Molecular Geometry Shape And Bond Angles Ammonia Youtube. Is Nh3 Polar Or Nonpolar Vsepr Theory Molecules Polar. Nh3 Lewis Structure Ammonia Youtube. Lewis Structure Nh3 Plus Dipoles Shape Angles And Formal Charge Youtube.
Nh3 Lewis Structure has 4 regions of electron thickness on all sides of the central nitrogen atom (3 bonds and one lone pair). These have a tetrahedral shape. These have a tetrahedral shape. The ensuing molecular shape or structure is like trigonal pyramidal with H-N-H angles of 106.7°.
... Diagram , Mercury Outboard Wiring Diagram Schematic , Eukaryotic Cell Diagram With Labels , Thermostat Wiring Color Code , Fog Light Relay Wiring , ...
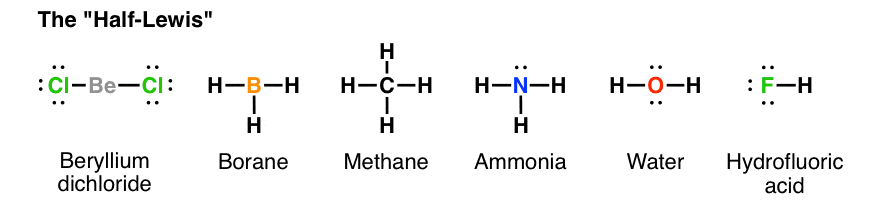
Lewis Structures. What are the Lewis structures of various compounds? 1. What is the Lewis structure for Water? 2. 3. What is the Lewis formula for ammonia (NH 3 )? 4.
When NH3 contributes its lone pair of electrons to BF3, it serves as a Lewis base in this diagram. When BF3 absorbs the lone pair of electrons that NH3 donates, it becomes a Lewis acid. This reaction fills BF3's vacant 2p-orbital, making boron sp3 hybridize where it was previously sp2 hybridized (as BF3).
NH3 Molecular Structure With the help of Lewis structure, we will understand electron geometry, polarity, and other properties of both polar molecules and non-polar molecules. The bonding pair of electrons, or those that build bonds, create the shape of the NH3 molecules.
Flag ShowMe Viewed after searching for: ... Lewis electron dot diagram for NH3
14+ Electron Dot Structure Of Nh3. Alternatively a dot method can be used to draw the nh3 lewis structure. We're going to do the lewis structure for nh3: NH3 Lewis and 3-D Structure- Dr. Sundin - UW-Platteville from people.uwplatt.edu Nitrogen has 5 valence electrons, but notice that nh4+ is a…
Welcome to Sarthaks eConnect: A unique platform where students can interact with teachers/experts/students to get solutions to their queries. Students (upto class 10+2) preparing for All Government Exams, CBSE Board Exam, ICSE Board Exam, State Board Exam, JEE (Mains+Advance) and NEET can ask questions from any subject and get quick answers by subject teachers/ experts/mentors/students.
NH3 Lewis Structure . Formal Charge on all atoms in NH4+ When ammonia forms a coordinate covalent bond with H+, NH4+ is formed. As we discuss above, nitrogen has a lone pair in the ammonia molecule. It has a tendency to share its lone pair with the electron-deficient species and form a coordinate covalent bond. Normally, the valency of nitrogen ...
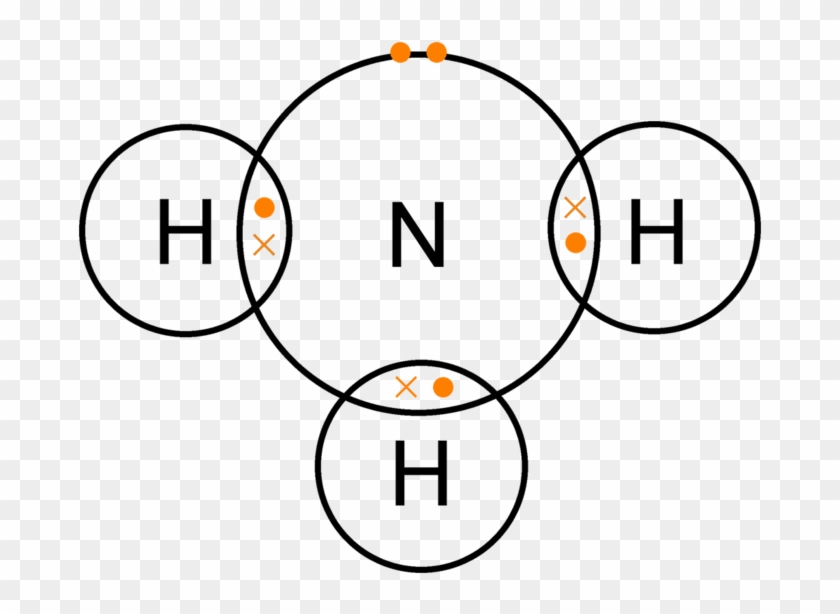
The Nitrifying Bacteria Cause What Is Commonly Called Dot And Cross Diagram Of Nh3 Free Transparent Png Clipart Images Download
Ammonia is a compound of nitrogen and hydrogen with the formula NH 3.A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. It is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to 45 percent of the world ...
Is Ammonia or NH3 Lewis Acid Or A Base? NH3, likewise identified as Ammonia, is a pungent-smelling gas substance that comprises one atom of Nitrogen and three hydrogen atoms. Ammonia has a low boiling temperature level at -33 degrees Celsius as well as is lighter than air. Often students guess about whether NH3 is an acid or Base.
The lone pair in NH3 is given to the H+ ion to do a brand-new N-H bond. Now, over there are 4 N-H bonds around nitrogen atom. See the reaction that ammonia and also HCl. Lewis framework of NH4+ has a +1 charge on nitrogen atom. Therefore is it a secure structure? First, we should know, atoms in a lewis structure can contain charges.

Give The Lewis Structures For The Following Compounds Include All Bonding Pairs And Non Bonding Lone Pairs A Nh3 B H2s C Co2 D Sih4 E C2h6 Study Com

Ammonia 2d Dot Cross Dot Cross Diagram For Ammonia Png Image Transparent Png Free Download On Seekpng
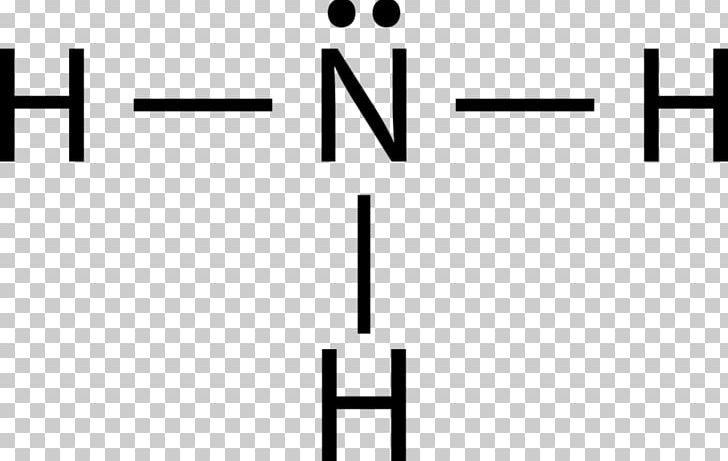
Lewis Structure Ammonia Lone Pair Ammonium Lewis Pair Png Clipart Ammonia Ammonium Angle Area Black Free
















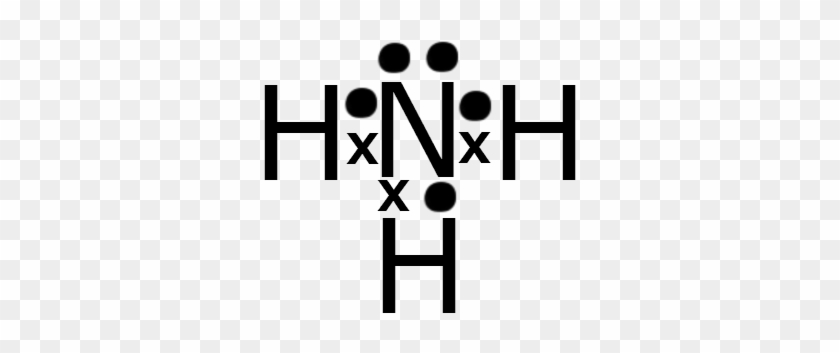


0 Response to "38 lewis diagram for nh3"
Post a Comment