39 lewis dot diagram for nh3
The lewis structure that is also called an electron dot structure, sa reprsentation de Lewis est la suivante :. Log in with Schéma de lewis nh3. Already have an account. Menu Categories. There are three single bonds schéma de lewis nh3 one lone pair of electrons in NH3 molecule. Ammonia is a colorless compoundused in making fertilizers. NH3 + H+ ——> NH4+ Lewis Structure. Lewis Structure is a simplified arrangement and presentation of the electrons present in the valence shell of a molecule. A Lewis Structure is a depiction of the arrangement of electrons in the standalone atoms of an element. In the Lewis Structure, electrons are depicted as dots.
Ammonia (NH3) is a commonly tested Lewis structure due to it's widespread use in agriculture as a fertilizer. It also is a good example of a molecule with a ...Oct 25, 2016 · Uploaded by Wayne Breslyn
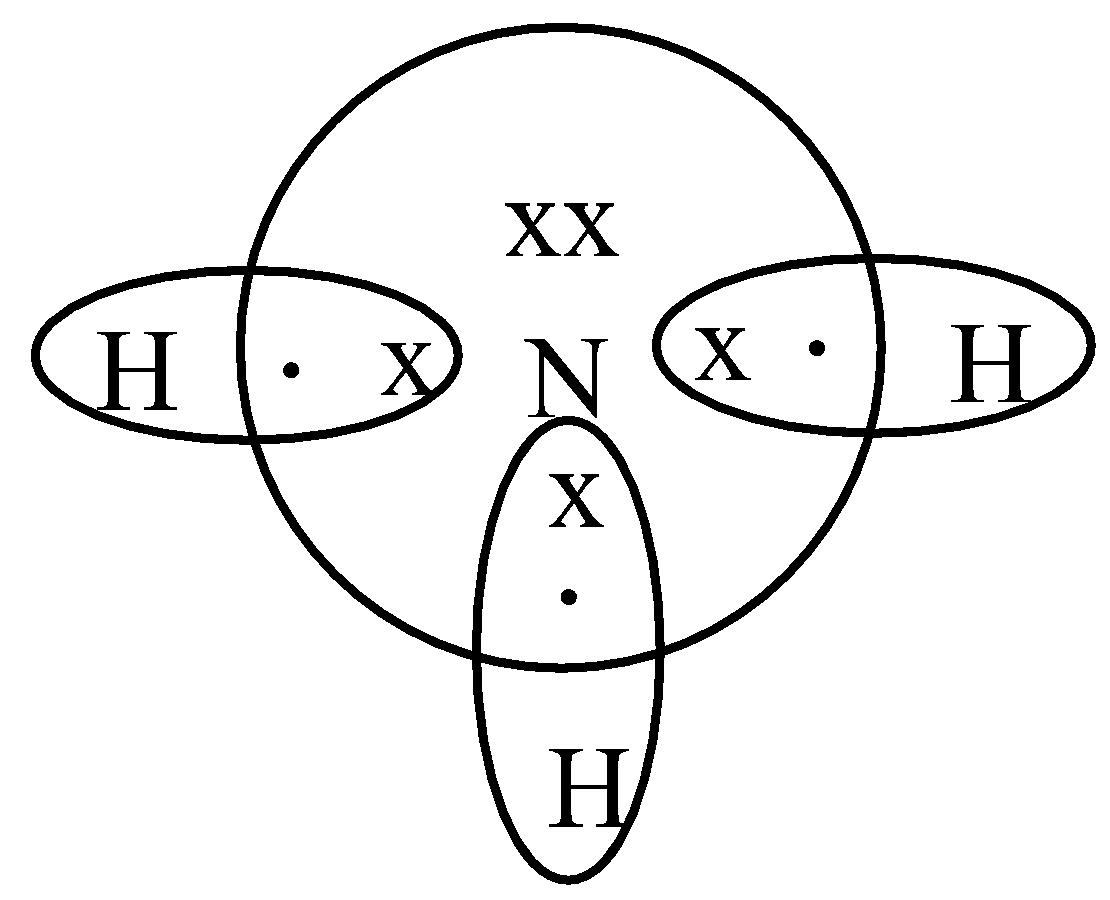
Lewis dot diagram for nh3
A lewis electron dot diagram or electron dot diagram or a lewis diagram or a lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Weve used all 26 valence electrons. Bond angle of a NI3 molecule. Get the detailed answer. Are molecular or coval. Lewis dot ammonia diagram structure structures ammonium molecule nh4 draw electron nh3 reactions represented chemical ph3 questions example upvoter sharers. Since it is bonded to only one carbon atom it must form a double bond. Alternatively a dot method can be used to draw the lewis structure of NH3. Lewis dot structure of ammonia. N 7 and H 1. The molecular weight of Ammonia is 17 g/mol. It is a colourless alkaline gas. Starting with the Lewis dot structure of Ammonia, Nitrogen has 5 valence electrons ...
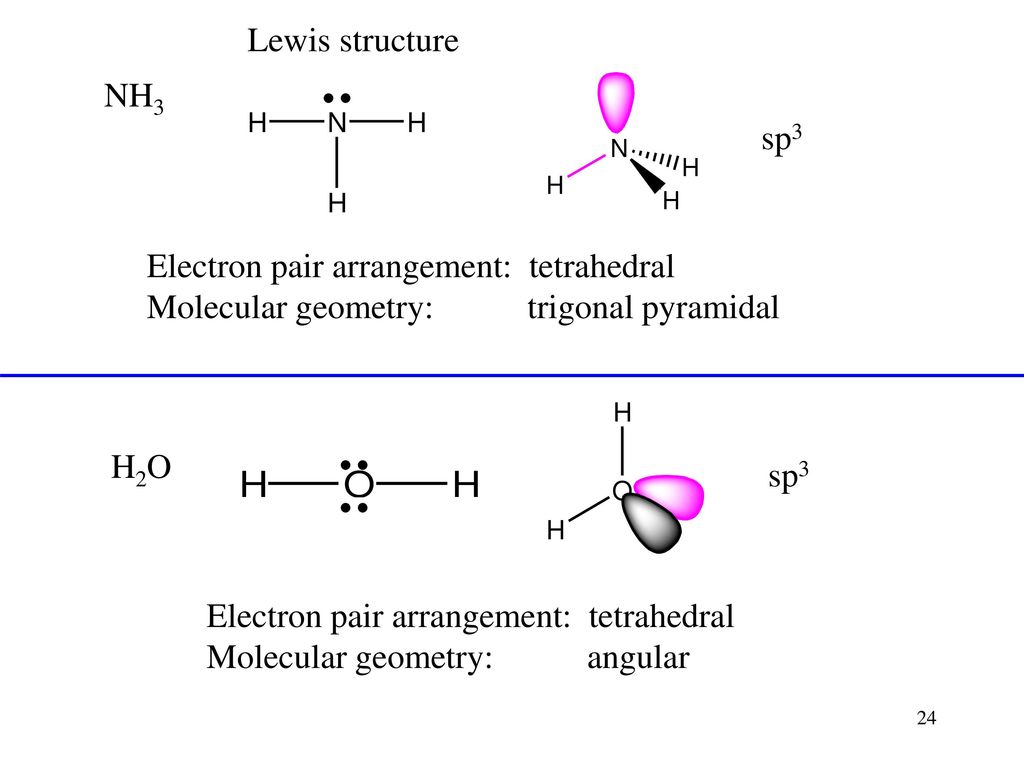
Lewis dot diagram for nh3. a) NH3, and H2S (correct) b) NH3, BF3, and H2S (BF3 has polar bonds, but is a nonpolar molecule) c) I2 only d) BF3 Chemistry 121 What is the lewis dot structure of C2H5F? When NH3 contributes its lone pair of electrons to BF3, it serves as a Lewis base in this diagram. When BF3 absorbs the lone pair of electrons that NH3 donates, it becomes a Lewis acid. This reaction fills BF3's vacant 2p-orbital, making boron sp3 hybridize where it was previously sp2 hybridized (as BF3). Lewis Dot Diagram Of Nh3 The Lewis structure of ammonia NH3 would be three hydrogen atoms bonded to a nitrogen atom in the middle with a lone pair of electrons. Ammonia NH3 or H3N CID 222 - structure chemical names physical and chemical properties classification patents literature biological activities safety. It also is a good example of a ... We can illustrate the formation of a water molecule from two hydrogen atoms and an oxygen atom using Lewis dot symbols: The structure on the right is the Lewis electron structure, or Lewis structure, for H 2 O. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. Moreover, by sharing a bonding pair with oxygen ...
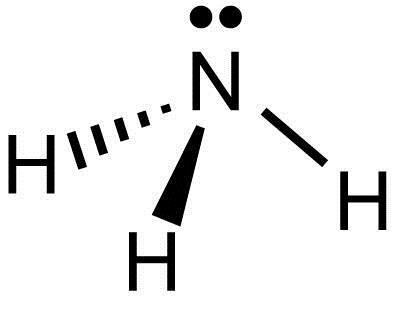
Lewis dot structure for: CH3Cl; H2Te; NH3; TeI2; C2H6; C3H6; C2H5Cl; HCN; CS2; C2H5OH; N2; H2NCH2Br; The correct formula of the compound whose name is hexaamminechromium(III) nitrate is … The Be-F Bond In BeF, Is O 180 109.5 O 109.50 120 Nonpolar Polar The Molecule Bef, Is Nonpolar Polar . A H 2 S has four electron pairs around the sulfur ... Alternate lewis dot structure of water. 10+ Nh3 Lewis Structure. This is the reason why ammonia acts as a lewis base, as it can donate those electrons. (1) you have two chlorine atoms. Bonding and structure in covalent compounds. Electron dot structures or lewis dot formula can be drawn if the molecular formula of the compound is known. Nh3 lewis dot structure. The Lewis Dot Structure for NH3 Ammonia is shown above. Formed when the atoms need to form an octet but both. NH 3 Ammonia is a commonly tested Lewis structure. If the species is an ion add or subtract electrons corresponding to the charge of the ion. The electron-dot structure of NH3 places one set of nonbonding electrons in the valence shell of the nitrogen atom. that means there are three bonded atoms and one single pair for a coordination number of four surrounding the nitrogen, the same occurs in water to. along with the Lewis dot structure for ammonia, NH3. the non bonding atoms are ...
May 5, 2018 — The Lewis structure of ammonia, NH3 , would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons ...1 answer · Have a look here... Explanation: The Lewis structure of ammonia, NH3, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone ... What is the Lewis dot symbol of chlorine? For example, when two chlorine atoms, each with 7 valence electrons, come together to form a diatomic chlorine molecule, the Lewis structure shows that there will be a sharing of two electrons between the two chlorine atoms which allows both chlorine to be surrounded by 8 electrons….Lewis Dot Structures. A lewis dot structure is also called a lewis structure, a lewis dot diagram, an electron dot structure note the lone pair (dots without bonds) on top of p, just like for n in the previous example for nh3. Ch3 ch2 ch ch2 ch3 nhch3. So you get carbon surrounded by single bonds to h. Ammonia lewis structure contains three N-H bonds and one lone pair on nitrogen atom. Lewis structure of NH3 can be drawn by using valence electrons of ...
0000005679 00000 n 1.A Lewis electron-dot formula (Lewis structure) is identical to a structural formula. :[29], Treatment of the double salt with hydrogen sulfide gives CsCl:[29], High-purity CsCl is also produced from recrystallized It is closely related to Lewis structure and it is an integral part of organic chemistry.
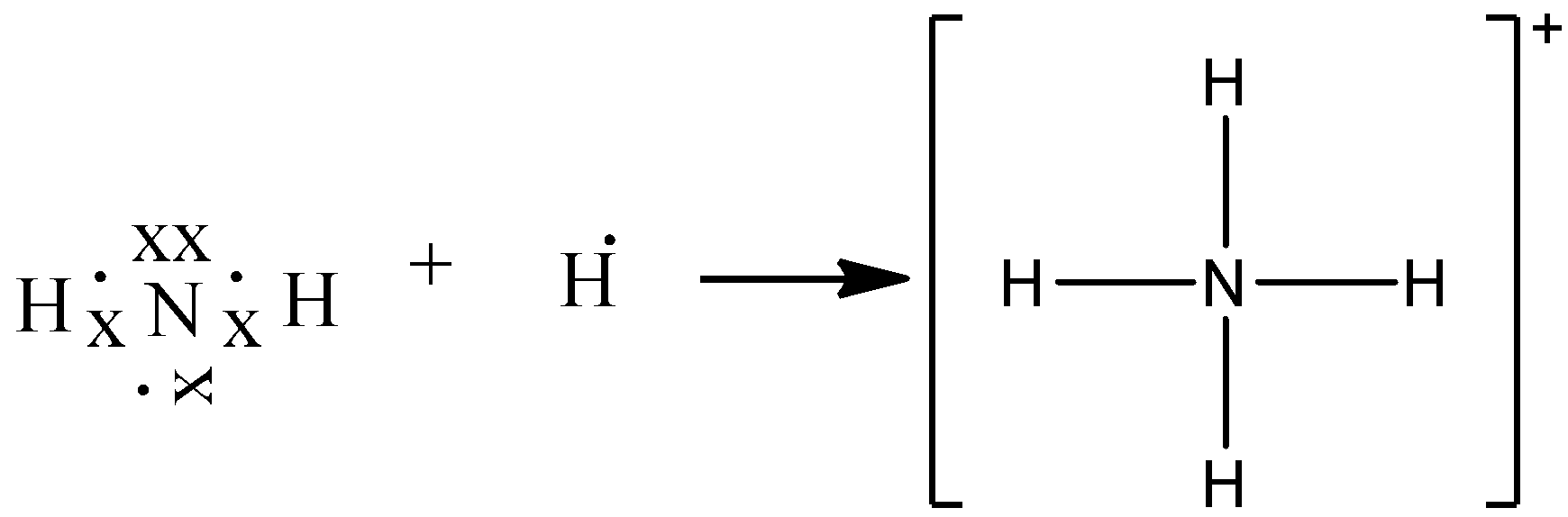
The lewis dot structure for ch3ch2nh3+ this problem has been solved! When considering the best stability for octet lewis structures, it is a matter of physics as we consider the stability of the electric charge: The lewis dot structure for ch3ch2nh3+ this problem has been solved! Using equation 8.5.1, the formal charge on the nitrogen atom is ...
In this blog post, we are going to find out if NH3 is polar or nonpolar. NH3 Polarity. To know the polarity and other properties of any molecule, it is vital first to understand its Lewis structure. We have previously shared a detailed blog on the NH3 Lewis structure that you can check out for a quick revision of its lewis dot structure.
Ammonia has 4 covalent bonds, 1 with each Hydrogen atom (although one of the bonds is dative due to the fact that nitrogen gives two electrons to one of the ...3 answers · 2 votes: Electrons of N is shared by H to complete its duplet and elctrons of H is shared by N to ...
Every Lewis dot diagram makes use of 16 valence electrons and fills the outer shell of every atom. Nevertheless, the atoms could also be organized and bonded otherwise. For the C3H4 Lewis structure, calculate the complete variety of valence electrons for the C3H4 molecule (C3H4 has 16 valence electrons). . In addition to, what is […]
Lewis structures (also known as Lewis dot structures or electron dot structures) are diagrams that represent the valence electrons of atoms within a molecule. These Lewis symbols and Lewis structures help visualize the valence electrons of atoms and molecules, whether they exist as lone pairs or within bonds.

Ammonia Molecule Nh3 Lewis Dot Cross Electronic Diagram Covalent Bonds Ball Stick Space Filling 3d Models Boiling Point Melting Point Doc Brown S Chemistry Revision Notes
Nh3 Lewis Structure Dipole. October 08, 2021. Ljmong8psaejbm. Nh3 Molecular Geometry Shape And Bond Angles Ammonia Youtube. Is Nh3 Polar Or Nonpolar Vsepr Theory Molecules Polar. Nh3 Lewis Structure Ammonia Youtube. Lewis Structure Nh3 Plus Dipoles Shape Angles And Formal Charge Youtube.
Chemical Formula Total Number of Valence Electrons Lewis Dot Structure CH4 NH3 CF4 CO2 BF3 C4H6 H2O H2 Cl2 PF3 HF HCl N2 C2H4 Title. Electron Dot Structure or Lewis Dot Diagram Gilbert Lewis A notation showing the valence electrons surrounding the atomic symbol. View Lewis Structure worksheet1doc from CHEM 525-01 at Bishop Fenwick High School.
NH3 Lewis Structure, Geometry, and Hybridization. Ammonia is the simplest binary hydride made up of nitrogen and hydrogen denoted by its chemical formulae as NH3. It is a stable pnictogen hydride where all the atoms are covalently bonded to achieve a reactive state. Ammonia is lighter than the air, colorless, and pungent in smell.
Ammonia or NH3 has actually a complete of 8 valence electrons. NH3 Lewis Structure. The Lewis framework of a molecule helps recognize the electron geometry, molecular geometry, polarity and also other such properties v ease. The is a pictorial representation the the setup of valence electrons around the individual atom in the molecule.
BHow many electrons should be shown in the Lewis dot structure for hydrogen. Because hydrogen only needs two electrons to fill its valence shell it is an exception to the octet rule. Report an issue. 1 Nitrogen atom needs 3 electrons and all 3 Hydrogen atoms need 1 more electron to get stable.
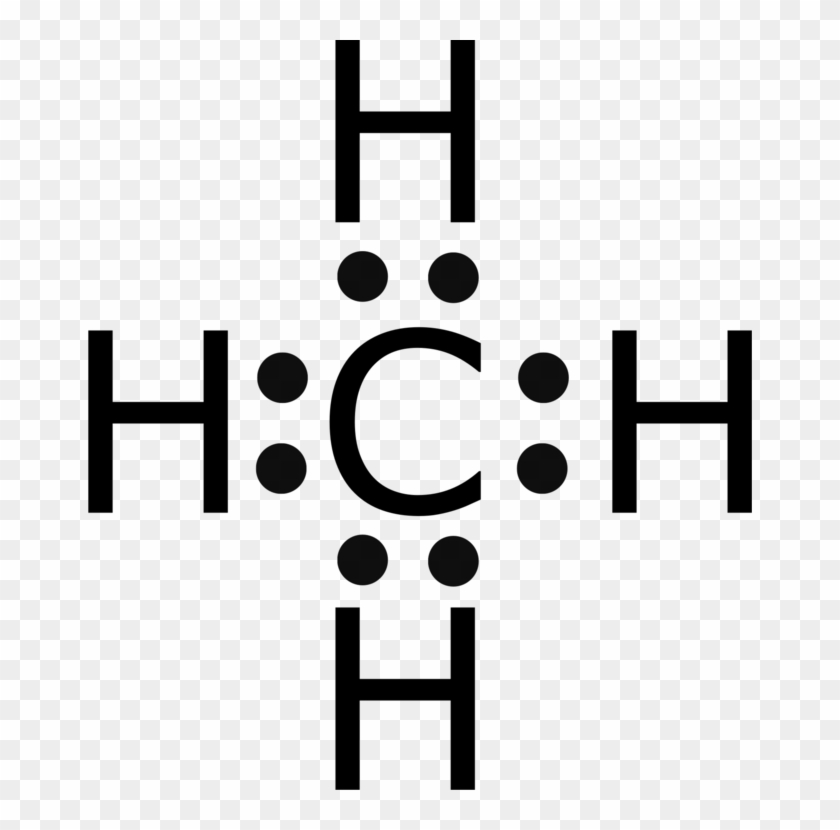
Lewis Structure Methane Electron Atom Hydrogen Lewis Dot Diagram Of Methane Free Transparent Png Clipart Images Download
How To Draw The Nh4 3po4 Lewis Dot Structure Ammonium Phosphate Youtube . How To Write The Net Ionic Equation For Nh4 2so4 Koh K2so4 Nh3 H2o Youtube . How To Find The Number Of Atoms In Nh4 2so4 Ammonium Sulfate Youtube . Buy Ammonium Sulfate Pure Online Scientific Store .
What is the Lewis structure of NH3? Why ammonia acts as a Lewis base because it can donate those electrons. The (NH3) molecule features a trigonal pyramidal shape as predicted by the valence shell electron pair repulsion theory (VSEPR theory) with an experimentally determined bond angle of 106.7°.
NH3 Lewis Structure, Molecular Geometry, Hybridization, Bond Angle & Shape. Ammonia is a colorless compound, used in making fertilizers. It is a stable hydride formed of one nitrogen and three hydrogen atoms. The molecule has a pungent smell. It can form an NH4+ ion by accepting a proton.
H2O"s Lewis dot Structure offers it plenty of unique nature mostly because of the two lone pairs on the central oxygen atom. This boosts electron-electron repulsion and therefore create a bent framework as opposed to CO2"s linear structure. This "bent" molecular structure gives it plenty of unique properties such together being polar.
14+ Electron Dot Structure Of Nh3. Alternatively a dot method can be used to draw the nh3 lewis structure. We're going to do the lewis structure for nh3: NH3 Lewis and 3-D Structure- Dr. Sundin - UW-Platteville from people.uwplatt.edu Nitrogen has 5 valence electrons, but notice that nh4+ is a…
The molecular weight of Ammonia is 17 g/mol. It is a colourless alkaline gas. Starting with the Lewis dot structure of Ammonia, Nitrogen has 5 valence electrons ...
Lewis dot ammonia diagram structure structures ammonium molecule nh4 draw electron nh3 reactions represented chemical ph3 questions example upvoter sharers. Since it is bonded to only one carbon atom it must form a double bond. Alternatively a dot method can be used to draw the lewis structure of NH3. Lewis dot structure of ammonia. N 7 and H 1.
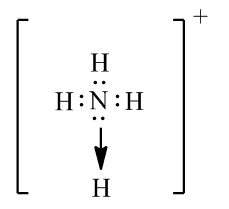
Write Is The Lewis Dot Structure Of Hno3 And Nh4 Using The Proper Steps Find A N And S Chemistry Topperlearning Com Eibpb699
A lewis electron dot diagram or electron dot diagram or a lewis diagram or a lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Weve used all 26 valence electrons. Bond angle of a NI3 molecule. Get the detailed answer. Are molecular or coval.

Best Answer Draw The Electron Dot Structure Of Ammonia Molecule And Show The Formation Of Ammonium Brainly In





















0 Response to "39 lewis dot diagram for nh3"
Post a Comment