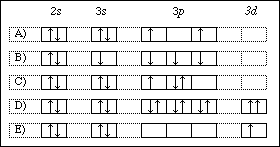
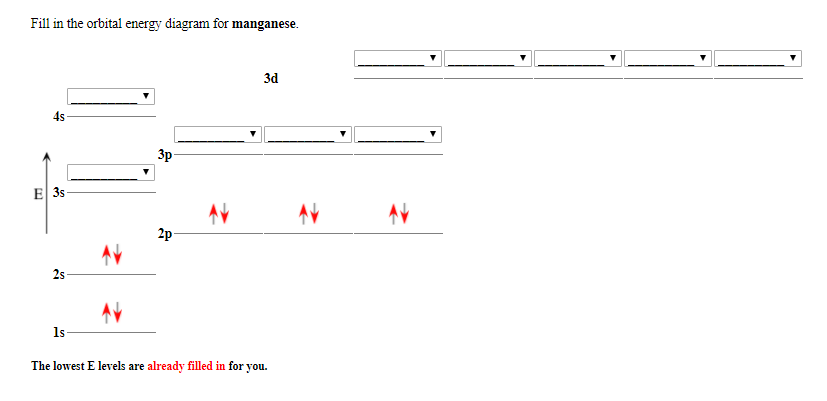
40 orbital diagram for manganese
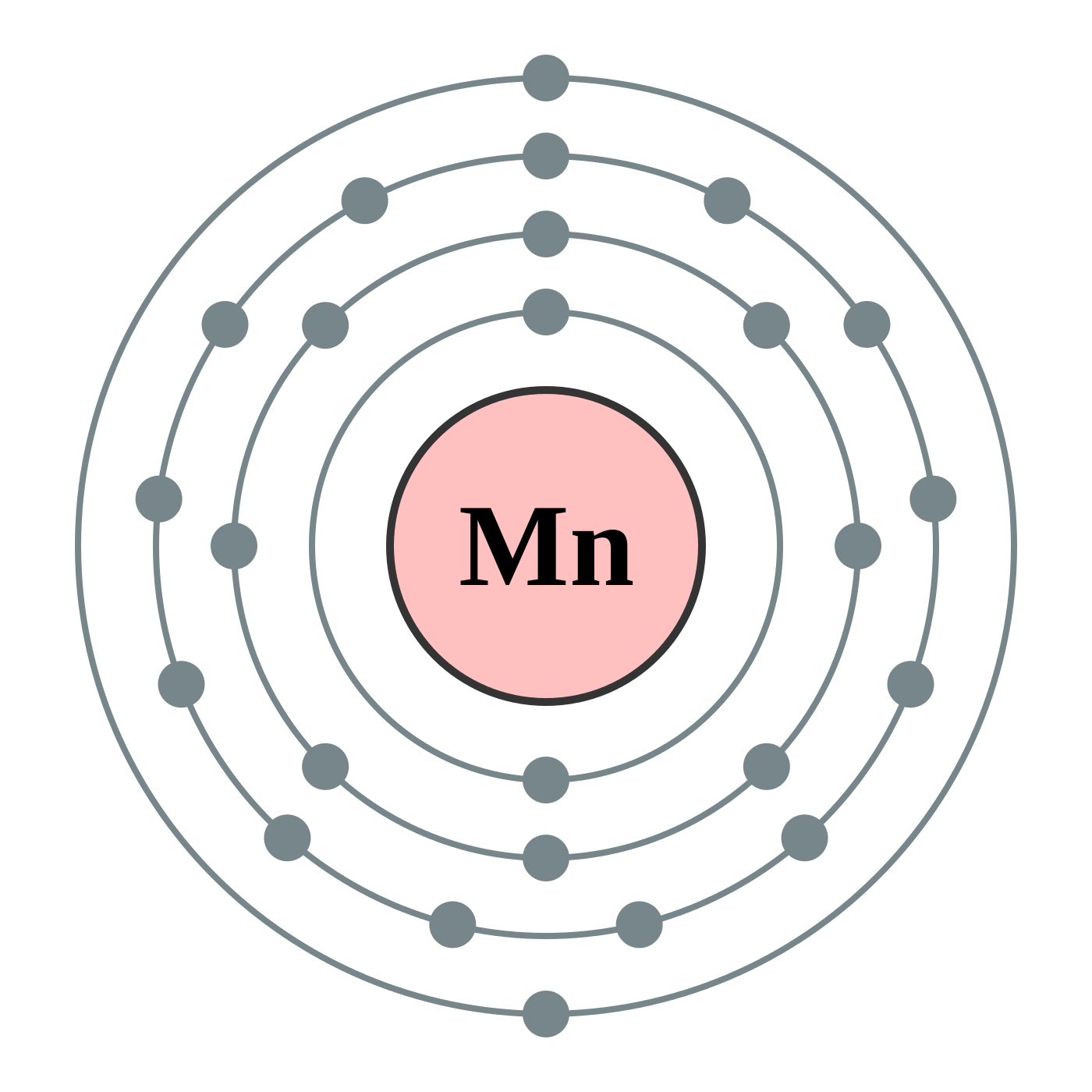
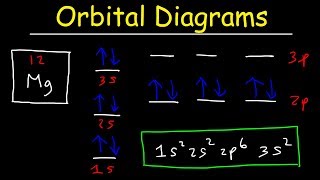
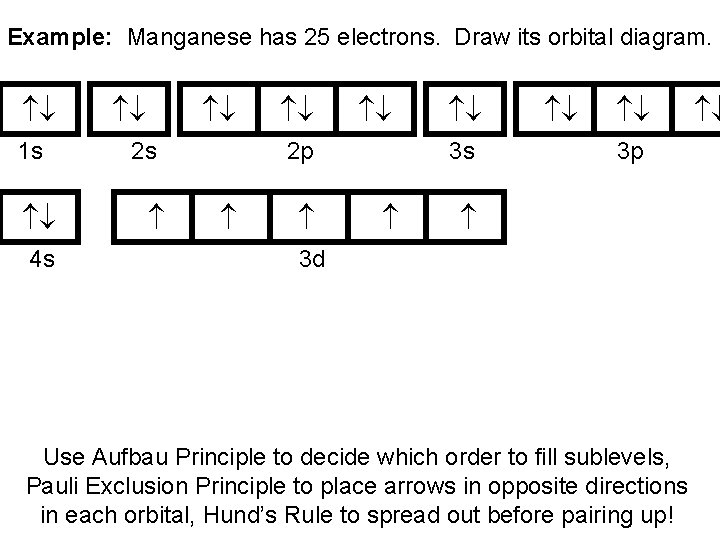
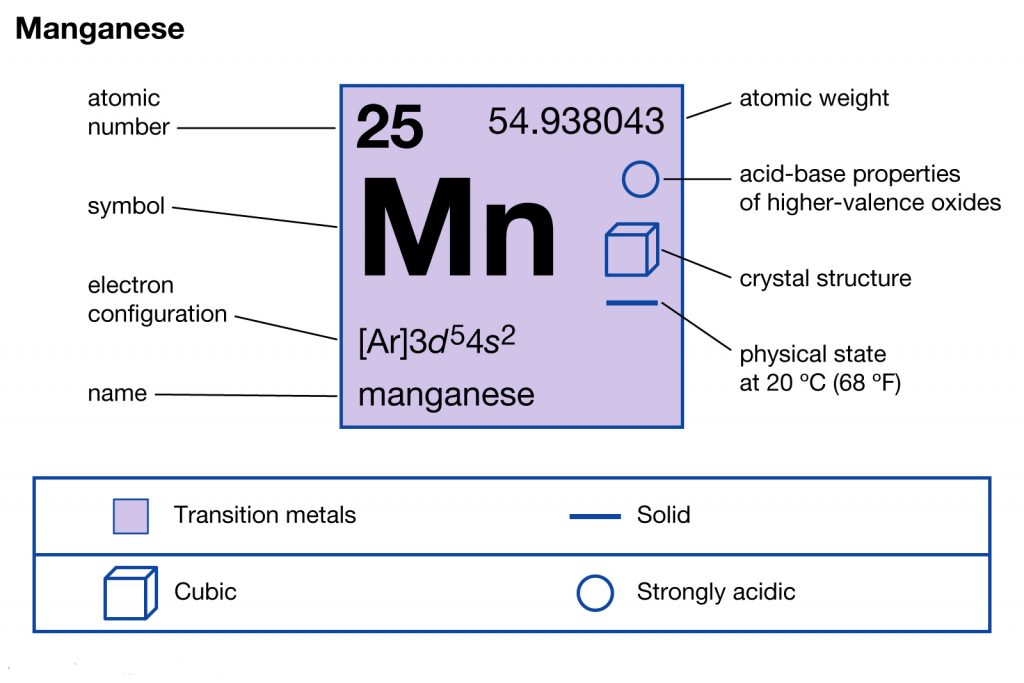
manganese mn chemicalaid manganese mn has an atomic mass of 25 find out about its chemical and physical properties states energy electrons oxidation and more. Hf Molecular Orbital Diagram – Orbital Diagram For Fluorine Awesome 0d Mos2 2d G. arrangements of electrons in the orbitals of an atom is the orbital diagram the electron configuration and the energy diagram all three ways are useful the next atom is helium with 2 electrons so the second electron could go into the 1s orbital with the ... Manganese (Mn) has an atomic mass of 25. Find out about its chemical ... Electron Configuration, [Ar] 4s2 3d5. 1s2 2s2 2p6 3s2 3p6 4s2 3d5. Orbital Diagram.Atomic Number: 25Symbol: MnAtomic Weight: 54.938045 Isotopes
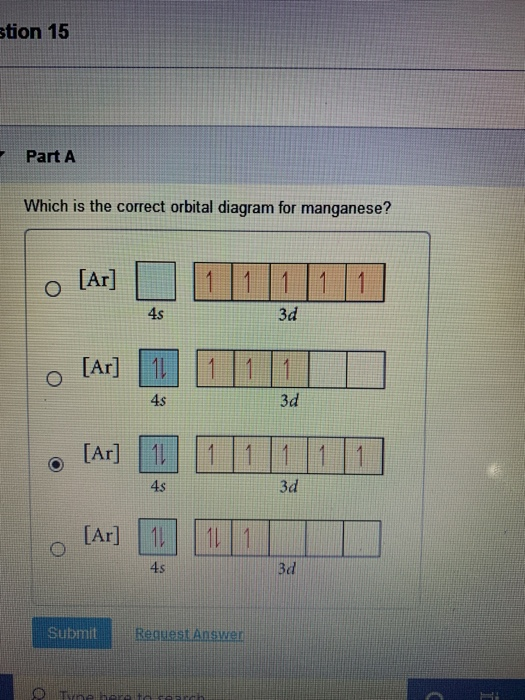
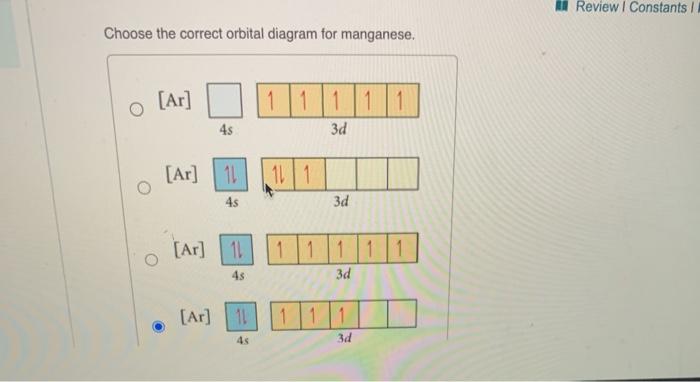
1 answerThe atomic number of Manganese (Mn) is 25. Thus its electronic configuration is [Ar]4s23d5 or. 1s22s22p63s23p64s24d5. Thus answer is option C.

Orbital diagram for manganese
Draw the orbital diagram diagram for manganese. Nov 01, 2021 · Orbital diagram of Manganese (Mn) 26: Orbital diagram of Iron (Fe) 27: Orbital diagram of Cobalt (Co) 28: Orbital diagram of Nickel (Ni) 29: Orbital diagram of Copper (Cu) 30: Orbital diagram of Zinc (Zn) 31: Orbital diagram of Gallium (Ga) 32: Orbital diagram of Germanium (Ge) 33: Orbital diagram of Arsenic (As) 34: Orbital diagram of Selenium ... Feb 08, 2015 · Explanation: Oxidation States. +2,3,4,6,7. Electrons Per Shell 2 8 15. Electron Configuration [Ar] 4s2 3d5. 1s2 2s2 2p6 3s2 3p6 4s2 3d5. Answer from: angelinaissoocp33868. SHOW ANSWER. The correct answer is Option D. Explanation:
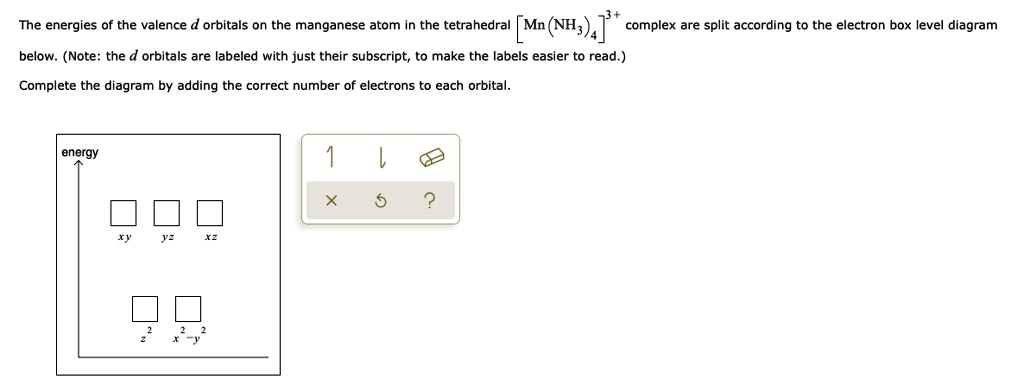
Orbital diagram for manganese. 29 Jul 2016 — The electron configuration of manganese, atomic number 25, is 1s2222p63s23p63d54s2 . The diagram below represents the electron configuration ...1 answer · Refer to the explanation. Explanation: The electron configuration of manganese, atomic number 25, is 1s2222p63s23p63d54s2. The diagram below represents ... So I need someone to check some of these, so I might crosspost this to other subreddits, if you know any, please do. Or if you are an expert yourself, please correct me if there's any mistakes. But I did watch Dr. Stone in an *Anime Streaming website*, I posted some interesting comments in the discussions of Dr. Stone Episodes. I will post them in a chronological order with the matching episodes. Although, I think it's a bad idea to post this in a whole one post. Because no one gonna read it t... Mn (Manganese) is an element with position number 25 in the periodic table. Located in the IV period. Melting point: 1244 ℃. Density: 7.44 g/cm 3 . Electronic configuration of the Manganese atom in ascending order of orbital energies: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 5. Orbital energy diagrams showing the possible ways that Mn(IV) can add two electrons to its e * g orbital set. Accepting two electrons in one orbital is more ...
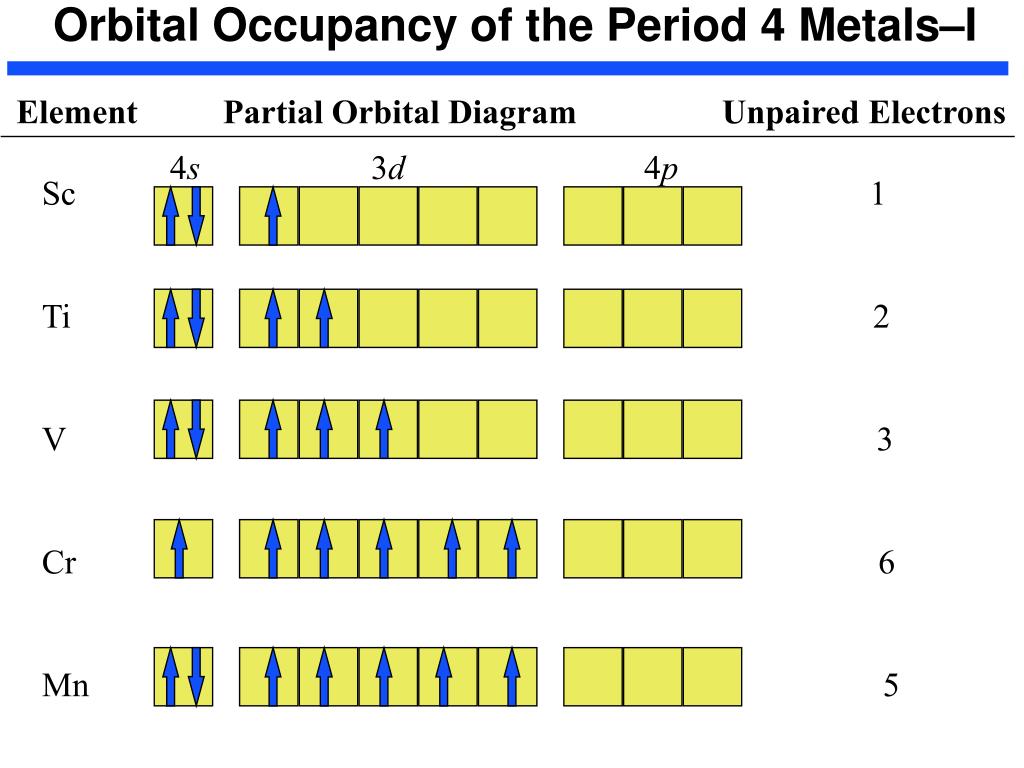
So if you look at the periodic table, Manganese is going to have an electron configuration of one S 2. She was too two P six, three S two, three, P six 45. And ...1 answer · Top answer: You can determine the ground-state electron configuration of Manganese (Mn) by referring to the periodic table and locating the position of Mn in the ... So, in class we did a lab comparing the magnetic properties of the first row of transition elements (From Manganese to Zinc) as cations (all 2+), and one of the post-lab questions talks about the electron configuration and orbital diagram of each cation. I understand that for each one, the 4s orbital electrons will go away, rather than the 3d orbital electrons, but Copper is an exception. It's electron configuration is [Ar] 4s1 3d10, rather than [Ar] 4s2 3d9. But what about Cu2+? It's electron c... Version 2.0 and Part 7 of "Utopian Religion", "Utopia", or "Vispthinkingpat, Thinkflexsense, and Soundpat Religion" &#x200B; # Efficient vispthinkingpat combination of English language, numerical list format, and logic language or Vispenlogist Language: * Listology <-> List-types-ology <-> Indented-list-ology and Non-indented-list-ology and Numerically-ordered-list-ology and Bulletpoint-ordered-list-ology and Vispenlogistology * Combatology <-> Shield-from-enemy-ology... Hey! I’ve recently started studying chemistry as one of my university classes. I’m genuinely curious, is the definition of paramagnetism and diamagnetism a bit problematic? From definition, paramagnetic substances are those with unpaired electrons. The opposite goes with diamagnetic substances, whose electrons are all paired. I see that some elements follow this, like Manganese for example. It contains unpaired electrons and is thus paramagnetic. Why is it though that other elements don’t fo...
The electron configuration reveals that in the change from chromium to manganese, the electron was added to the outermost shell. The resulting configuration ... Feb 08, 2015 · Explanation: Oxidation States. +2,3,4,6,7. Electrons Per Shell 2 8 15. Electron Configuration [Ar] 4s2 3d5. 1s2 2s2 2p6 3s2 3p6 4s2 3d5. Answer from: angelinaissoocp33868. SHOW ANSWER. The correct answer is Option D. Explanation: Nov 01, 2021 · Orbital diagram of Manganese (Mn) 26: Orbital diagram of Iron (Fe) 27: Orbital diagram of Cobalt (Co) 28: Orbital diagram of Nickel (Ni) 29: Orbital diagram of Copper (Cu) 30: Orbital diagram of Zinc (Zn) 31: Orbital diagram of Gallium (Ga) 32: Orbital diagram of Germanium (Ge) 33: Orbital diagram of Arsenic (As) 34: Orbital diagram of Selenium ... Draw the orbital diagram diagram for manganese.




















0 Response to "40 orbital diagram for manganese"
Post a Comment