42 lewis dot diagram for so3
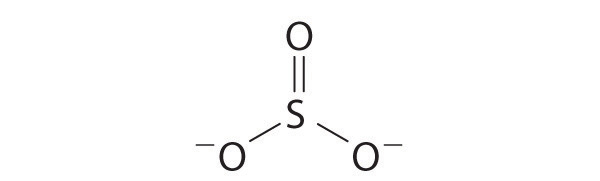
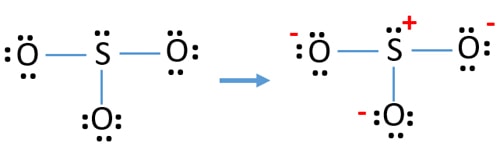
Are there pi bonds in sulfite Lewis structure? There is only one pi bond and it is located at between one pxygen atom and sulfur atom. Is lewis structure for so32- is different from lewis structure for so42-Yes. They are different. In SO 4 2-lewis structure, there are four oxygen atoms around the sulfur atom. Therefore number of valence ... Lewis Structure For So3 2-. The Lewis framework for SO32- is needs you come place more than 8 valence electrons on Sulfur (S).You can think you"ve acquired the exactly Lewis framework for SO3 at first. Remember, Sulfur is in period 3 and can hold much more than 8 valence electrons.You"ll desire to calculate the formal charges on every atom come ...
SO3 Molecular Geometry, Lewis Structure, and Polarity Explained. SO3 stands for Sulfur Trioxide. This is one of the most pollutant chemical compounds in the gaseous form. It is also a primary agent in the acid rain. The main use of this component is to make sulfuric acid for industrial purposes.
Lewis dot diagram for so3
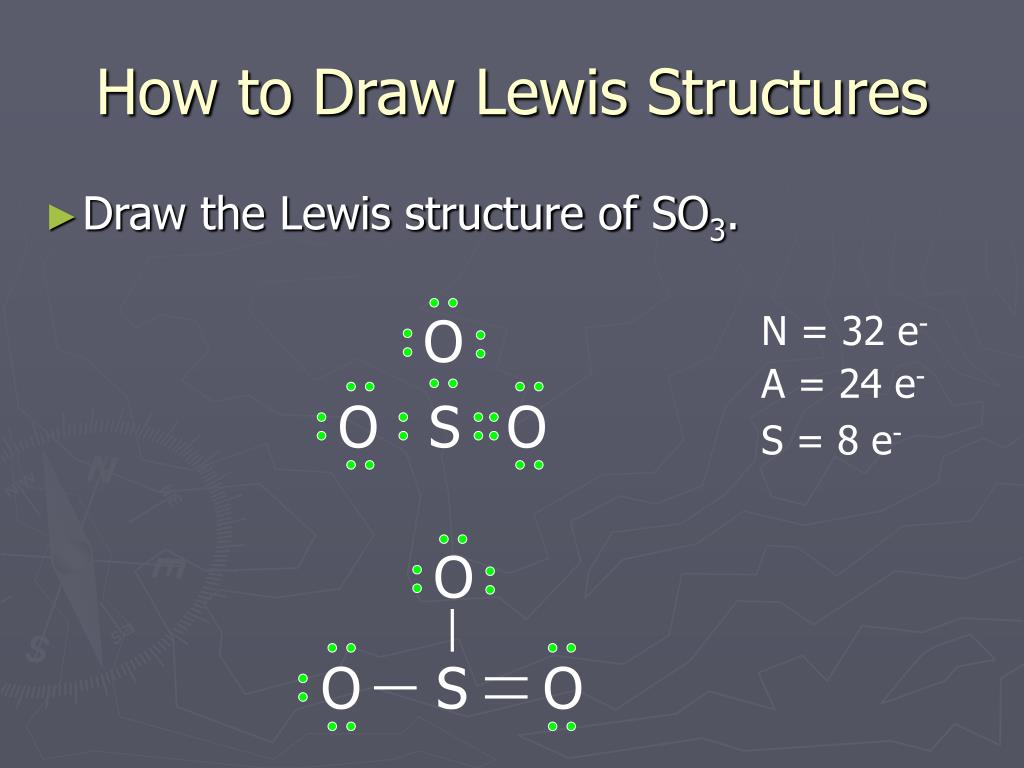
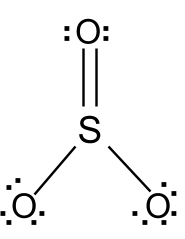
The Lewis Structure, or Lewis Dot Diagram, shows the bonding between atoms of a molecule and any electrons that may exist. The Lewis Structure for Li is Li with one dot to the right of the element. Chemistry questions and answers. 1. Draw the Lewis Dot Structure for the following: a. SO3 b. SO4-2 c. H2CO d. HCl 2.Using the Lewis Dot Structure from above, determine the Electronic Geometry (EG), Molecular Geometry (MG), and Polarity for each. Formula Electronic Geometry Molecular Geometry Polarity SO3 SO4-2. Drawing the Lewis Structure for SO 3 ( Sulfur Trioxide) SO 3 is the primary contributer to acid rain in the atomsphere. It is a form of pollution. SO 3 is named Sulfur Trioxide. There are 32 valence electrons available for the Lewis structure for SO 3. Be sure to check the formal charges for the Lewis structure for SO 3 .
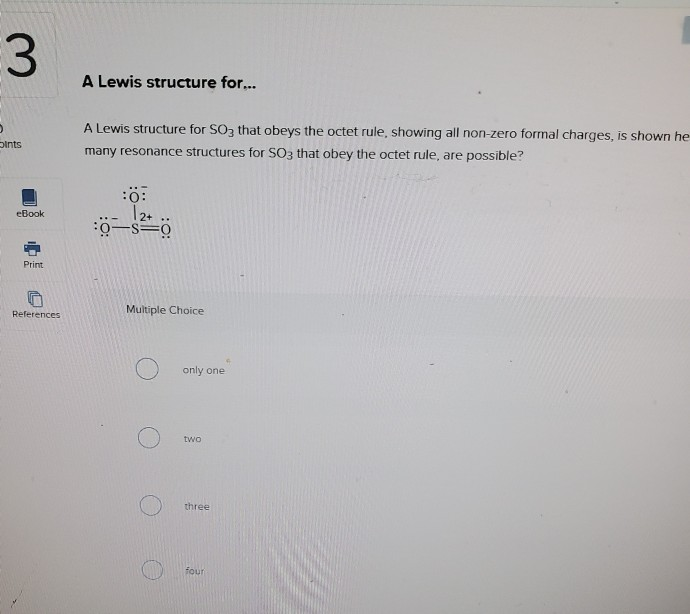
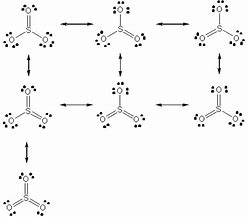
Lewis dot diagram for so3. 5:05How to draw the Lewis dot Structure of SO3 (sulfur trioxide)Sulfur is an exception to the octet rule due to the ...18 May 2021 · Uploaded by Chemistry 360 Lewis structure of SO3 The sulfur trioxide is a tetra atomic chemical molecule where both the sulfur and three oxygen molecules bond with an equal number of valence electrons. The diagram is drawn showing dots of valence electrons around the symbol of both sulfur and oxygen atoms with lines predicting bond formation. What is the Lewis dot structure of SO3? It is six for a single sulfur trioxide (SO3) molecule, where both sulfur and each oxygen atom need two valence electrons to stabilize their atom. Next, look for the number and type of bonds forming within a single sulfur trioxide (SO3) molecule. Draw the Lewis structure for the sulfur trioxide (SO{eq}_3{/eq}) molecule. Be sure to include all resonance structures that satisfy the octet rule.
How many bonds should be drawn in the Lewis-dot structure for SO3? apex users its defenatley 3. Is SO3 a Lewis base or Lewis acid? I think it is acid, because there is a question that asks the ... This problem has been solved! Write a single Lewis structure for SO3. Draw the Lewis dot structure for SO3. Include all lone pairs of electrons. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. The Lewis Dot Structure for SO 3:. SO 3 (sulfur trioxide), is a gaseous pollutant, and is used as sulfonation agent in the manufacturing of dyes and detergents, and some pharmaceutical products ... Website-http://www.kentchemistry.com/links/bonding/LewisDotTutorials/SO3.htmI quickly take you through how to draw the Lewis Structure of SO3 (Sulfur Trioxid...
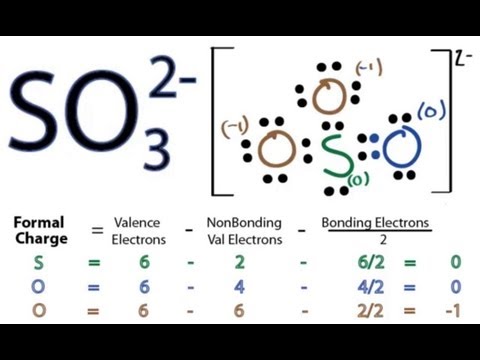
How to draw the Lewis Structure of SO3 sulfur trioxide - with explanationSulfur is an exception to the octet rule - it can handle up to 12 electronsCheck. So3-2 lewis structure. The Lewis Dot Structure for SO3 2-. Chemistry learning made easyThis tutorial will help you deal with the lewis structure and moleculargeometry of sulfite ion SO3 2-. simple procedure for drawing the SO3 lewis structure, so3 lewis electron dot structure, electrostatic potential, ESP, resonance structures of so3, Lewis structures of SO3| Lewis configuration of sulfur trioxide SO3, octet rule, SO3 Lewis structure, π bonds, 2π electrons, multiple bonds, stable resonance, resonance structures, so3 resonance or double bonds, lewis structures and the octet ... A step-by-step explanation of how to draw the SO3 2- Lewis Structure (Sulfite Ion). For the SO3 2- Lewis structure the total number of valence electrons ... Let's do the SO3 2- Lewis structure. For the SO3 2- compound, we have 26 total valence electrons, and that includes these two electrons up here--there are two extra valence electrons. So we have 26. Let's put the Sulfur at the center and the Oxygens around the outside. Put two electrons between the atoms to form chemical bonds. We've used 6.
Answer: Please read these instructions, if they do not make sense please PM me and we can set up an online session so I can facetime with you and get you squared away. Alright, here we go! Let's start off by differentiating between SO3 and SO3 2- SO3 is Sulfur Trioxide SO3²¯ is Sulfite They b...
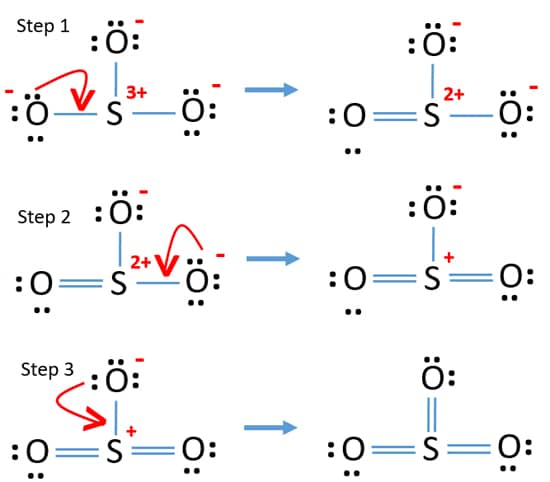
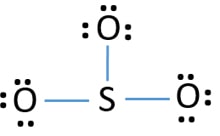
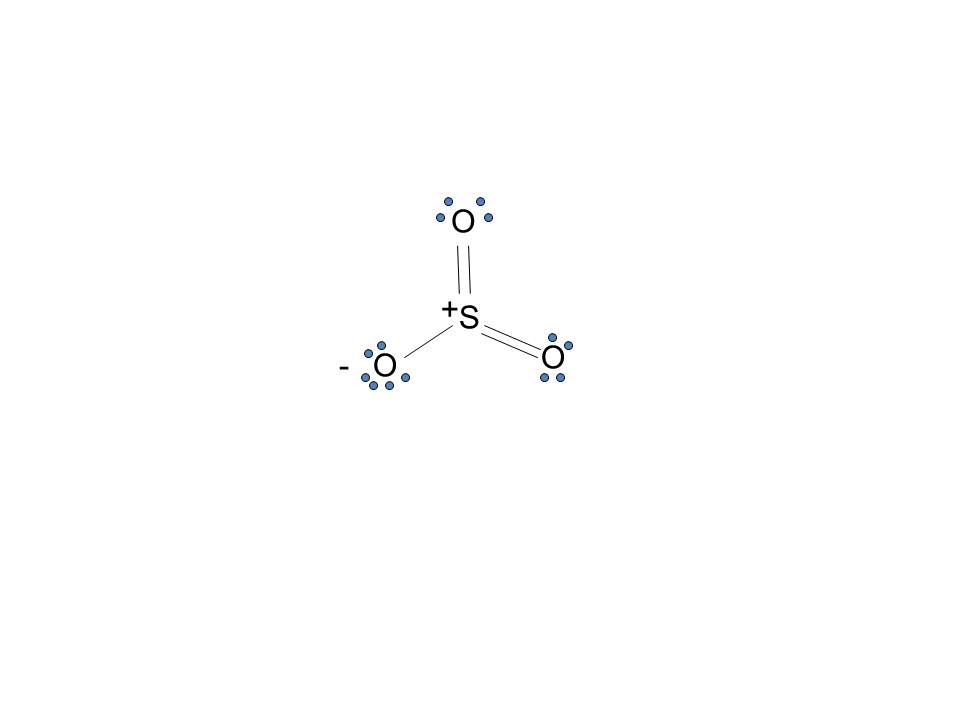
Lets do the so3 2 lewis structure. Lewis dot diagram for so3. So we have formal charges of zero for each of the atoms in so3. So 3 is the primary contributer to acid rain in the atomsphere. The lewis structure or lewis dot diagram shows the bonding between atoms of a molecule and any electrons that may exist.
5:49This chemistry video tutorial explains how to draw the lewis structure of SO3 also known as Sulfur Trioxide. It ...8 Jul 2020 · Uploaded by The Organic Chemistry Tutor
Lets do the so3 2 lewis structure. Lewis dot diagram for so3. So 6 minus 6 equals zero. 3 oxygens branch out. Alright here we go. So we have 26. For the so3 2 compound we have 26 total valence electrons and that includes these two electrons up here there are two extra valence electrons. I quickly take you through how to draw the lewis structure ...
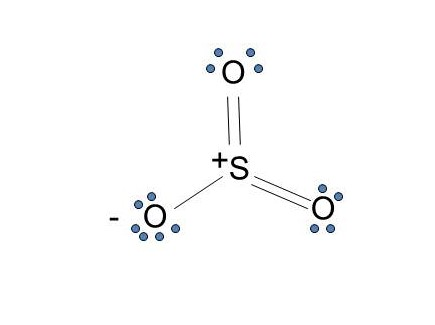
Lewis Diagram For Seo3. A Lewis dot structure for SeO3 is drawn with an Se in the center, with two lines connecting it to two Os and one double line connecting it to an O. The Os. Draw all possible resonance structures for the molecule selenium trioxide (SeO3) . The structure and arrows are given, simply add the remaining bonds and lone. (1 ...
Automatic procedure to construct Lewis dot structures, so32-, pi an d - Sulfite Ion SO3-2 Lewis structure, draw the lewis structure for the sulfite ion, Dot structure SO3-, so3 2-, SO32-,Sulfite,Ion,Lewis,dot,structure,hybridization,chemistry,bond,angle,drawing, ib, ap, a, SO3 2- Lewis Structure,Lewis Structure for SO3 2-,Lewis Structure,SO3 2-,SO3 2- Electron Dot Structure,Electron Dot ...
Lewis structures or electron dot structures - SO3 Lewis structure 1. Simple Procedure for writing Lewis Structures - Example: SO3 FOR CLICKABLE LINKS AND A DETAILED DESCRIPTION OF THE SIMPLE METHOD DESCRIBED BELOW PLEASE SEE PAGE 4 OF THIS PRESENTATION A simple procedure for writing Lewis structures is given in a previous article entitled "Lewis Structures and the Octet Rule".
So 6 minus zero minus 12 over 2; so 6 minus 6 equals zero. So we can write the formal charge for Sulfur as zero. So we have formal charges of zero for each of the atoms in SO3. That makes this the best Lewis structure for SO3. This is Dr. B., and thanks for watching.
4.7/5 (37 Views . 34 Votes) Drawing the Lewis Structure for SO3 (Sulfur Trioxide) It is a form of pollution. SO3 is named Sulfur Trioxide. There are 32 valence electrons available for the Lewis structure for SO3. Be sure to check the formal charges for the Lewis structure for SO3. Complete answer to this is here.
Lewis dot diagram for so3. Lets do the so3 2 lewis structure. So we can write the formal charge for sulfur as zero. For the so3 2 compound we have 26 total valence electrons and that includes these two electrons up here there are two extra valence electrons. It is a form of pollution. So we have 26.
Sulfur Trioxide (SO 3) Lewis Structure, Hybridization | Drawing Steps. Sulfur trioxide molecule contains one sulfur atom and three oxygen atoms. We will construct the lewis structure of SO 3 molecule by following VSEPR theory rules and considering stability of intermediate structures. Finally, after obtaining the lewis structure of SO 3, we can determine the hybridization of atoms.
Lewis dot diagram for so3. Let us consider the case of the sulfite ionthe chemical formula is so 3 2. It is a form of pollution. So 3 is named sulfur trioxide. So we have 26. For the so3 2 compound we have 26 total valence electrons and that includes these two electrons up here there are two extra valence electrons.
How to draw the Lewis Structure of SO3 (sulfur trioxide) - with explanationSulfur is an exception to the octet rule - it can handle up to 12 electrons!Check ...

Draw The Lewis Dot Structure For So32 Determine The Electron Geometry And Molecular Shape Of This Molecule Study Com
Answer (1 of 2): SO3 does not have the extra 2 electrons (SO3) 2- has. Therefore, the lewis dot structures will actually be very different. As shown below, The lewis structure of SO3 is being considered when no electrons will interfere with the synthesis of SO3, however this will change dramatic...
In the SO3 Lewis structure diagram, the sulfur atom can be the center atom of the molecule. As a result, central sulfur in the SO3 Lewis structure, with all three oxygen atoms arranged in trigonal planar geometry. Add valence electrons around the oxygen atom, as given in the figure. Step-3: Lewis dot Structure for SO3 generated from step-1 and ...
Lewis Dot Structure So3 2 Lewis dot of sulfur trioxide. Lewis dot diagram for so3. So3 lewis structure how to draw the lewis structure for so3 sulfur trioxide sign in. Lewis structures for so3. Lewis structure for so 3 sulfur trioxide a step by step tutorial. Discover what your dna has to say about your health with 23andme.
A step-by-step explanation of how to draw the SO3 Lewis Dot Structure (Sulfur trioxide).For the SO3 structure use the periodic table to find the total number...
Drawing the Lewis Structure for SO 3 ( Sulfur Trioxide) SO 3 is the primary contributer to acid rain in the atomsphere. It is a form of pollution. SO 3 is named Sulfur Trioxide. There are 32 valence electrons available for the Lewis structure for SO 3. Be sure to check the formal charges for the Lewis structure for SO 3 .
Chemistry questions and answers. 1. Draw the Lewis Dot Structure for the following: a. SO3 b. SO4-2 c. H2CO d. HCl 2.Using the Lewis Dot Structure from above, determine the Electronic Geometry (EG), Molecular Geometry (MG), and Polarity for each. Formula Electronic Geometry Molecular Geometry Polarity SO3 SO4-2.

Draw The Lewis Structure For So3 Determine The Number Of Electron Groups The Electron Geometry And The Molecular Shape Is It Polar Or Nonpolar Study Com
The Lewis Structure, or Lewis Dot Diagram, shows the bonding between atoms of a molecule and any electrons that may exist. The Lewis Structure for Li is Li with one dot to the right of the element.
Chemistry Class 11 Ncert Solutions Chapter 4 Chemical Bonding And Molecular Structure Part 3 Flexiprep

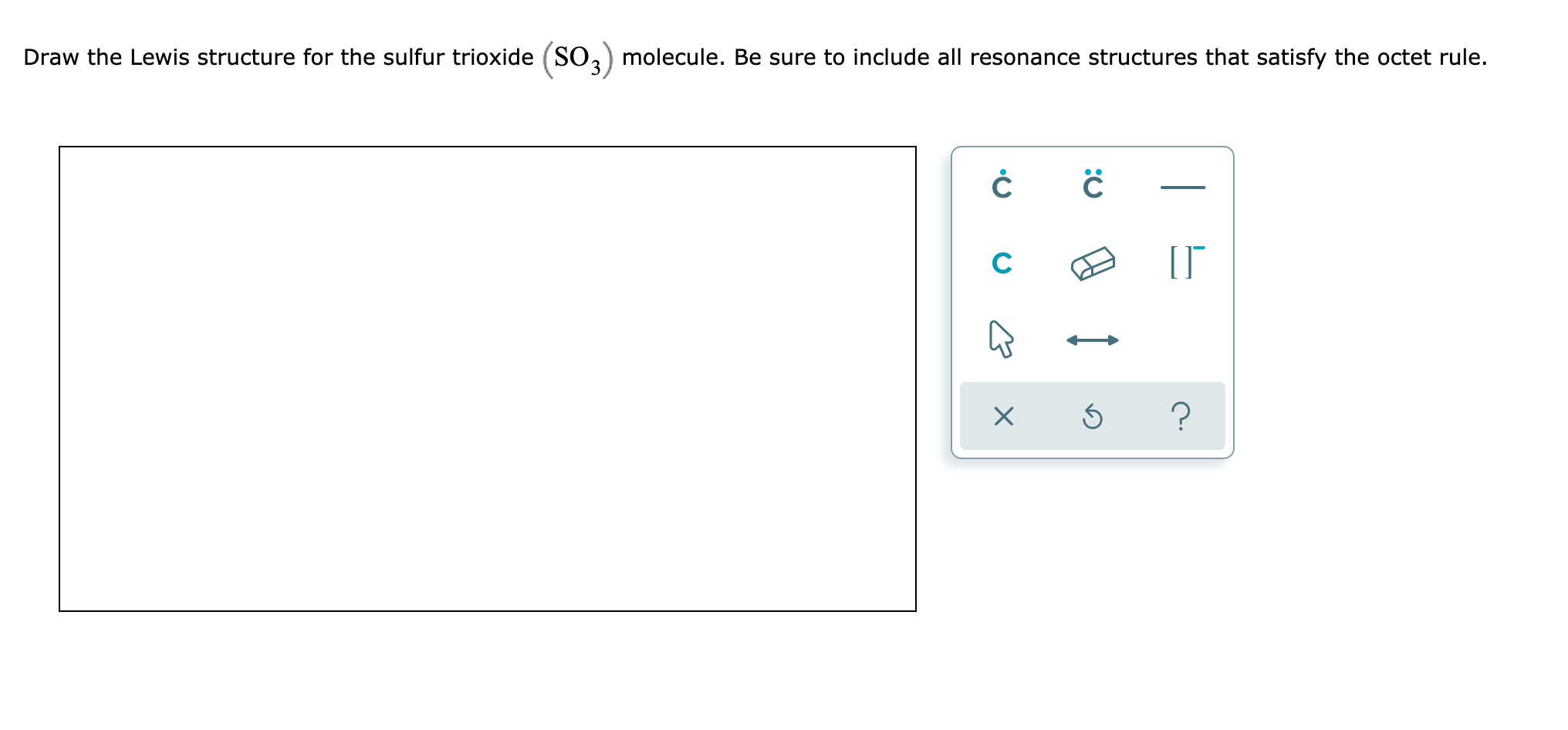
Draw The Lewis Structure For The Sulfur Trioxide So 3 Molecule Be Sure To Include All Resonance Structures That Satisfy The Octet Rule Study Com
Solved Draw Lewis Structure For The Following So2 Ph3 Ch2cl2 So3 2 C2f2 Cs2 Indicate If The Molecules Are Polar Or Non Polar So2 Ph3 Ch2cl2 So3 2 Course Hero
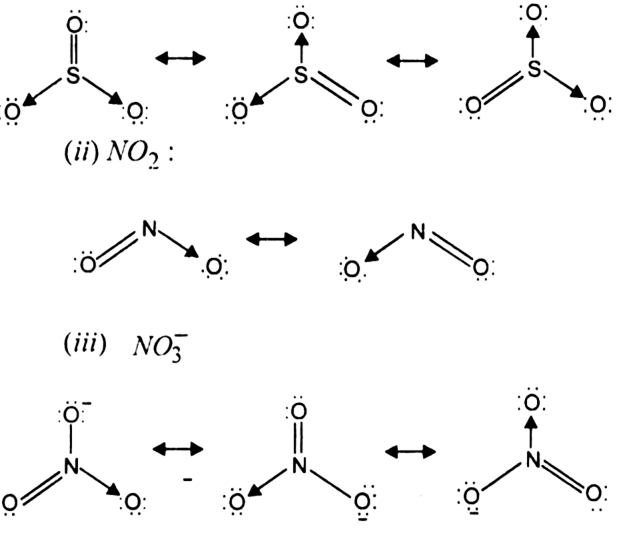
Write The Resonance Structures For So3 No2 And From Chemistry Chemical Bonding And Molecular Structure Class 11 Tripura Board
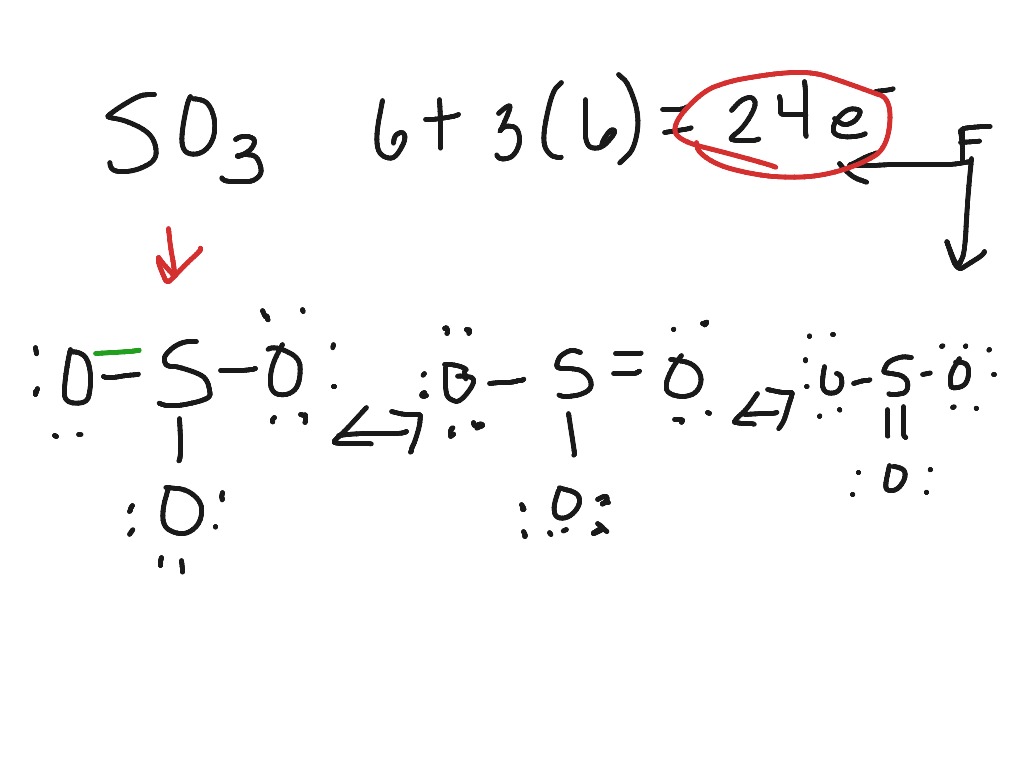



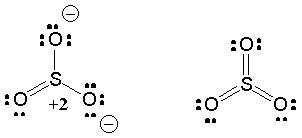









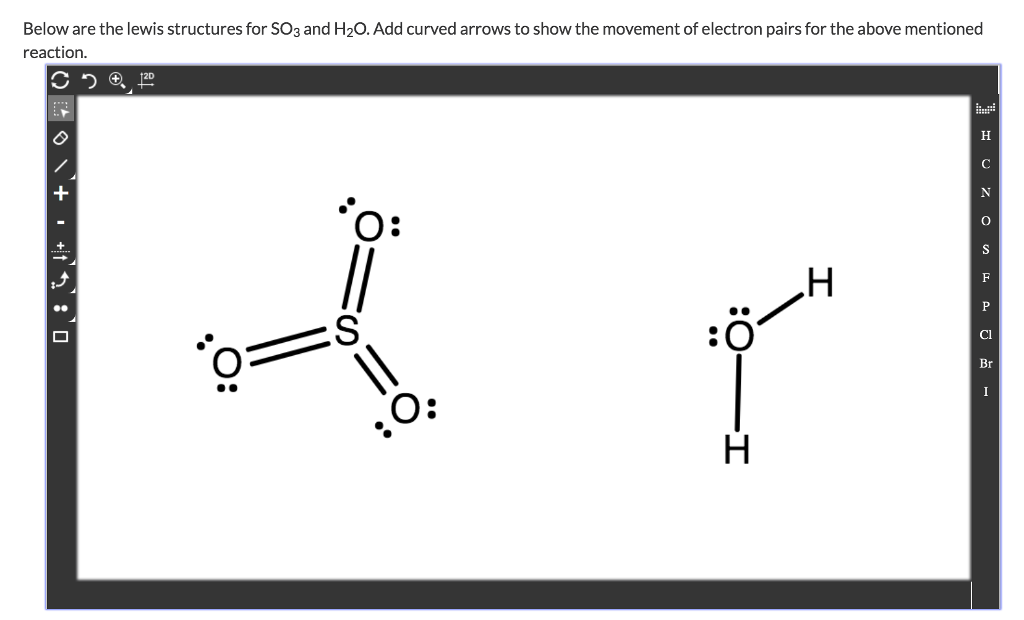

0 Response to "42 lewis dot diagram for so3"
Post a Comment