40 bohr diagram of fluorine
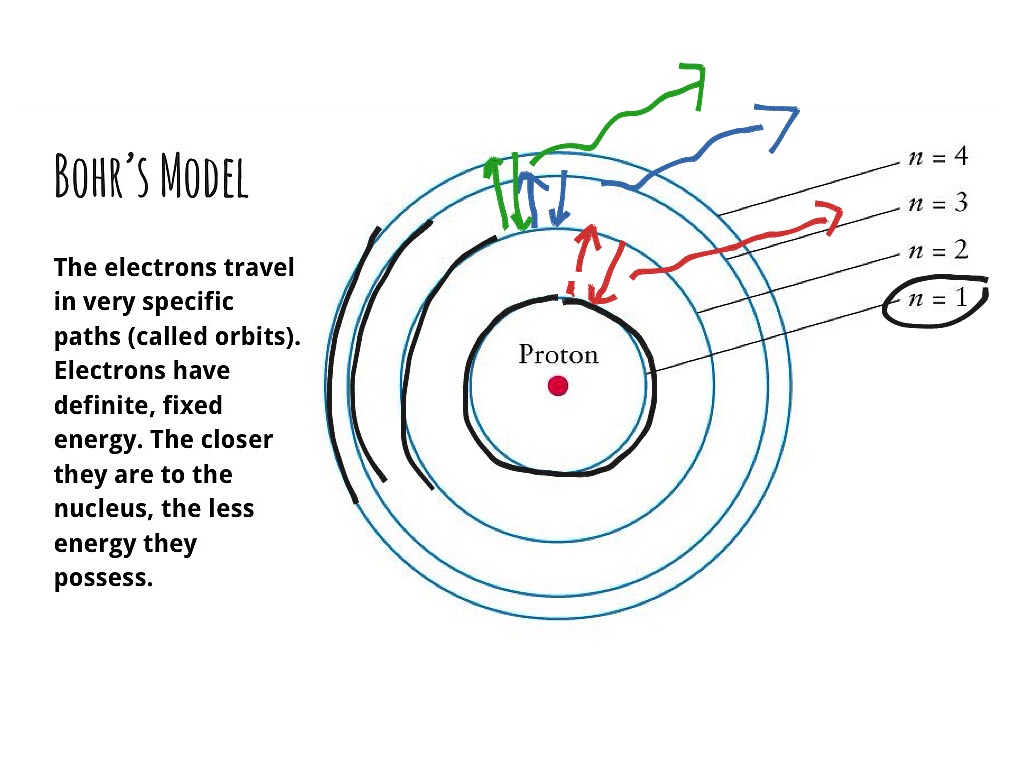
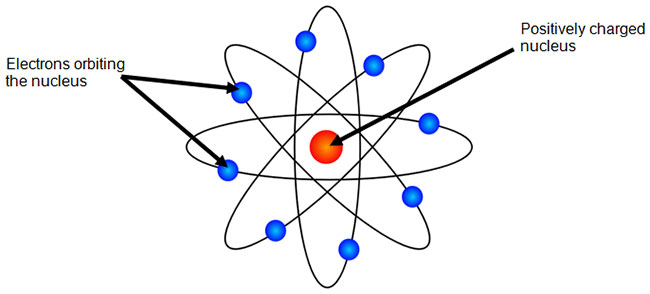
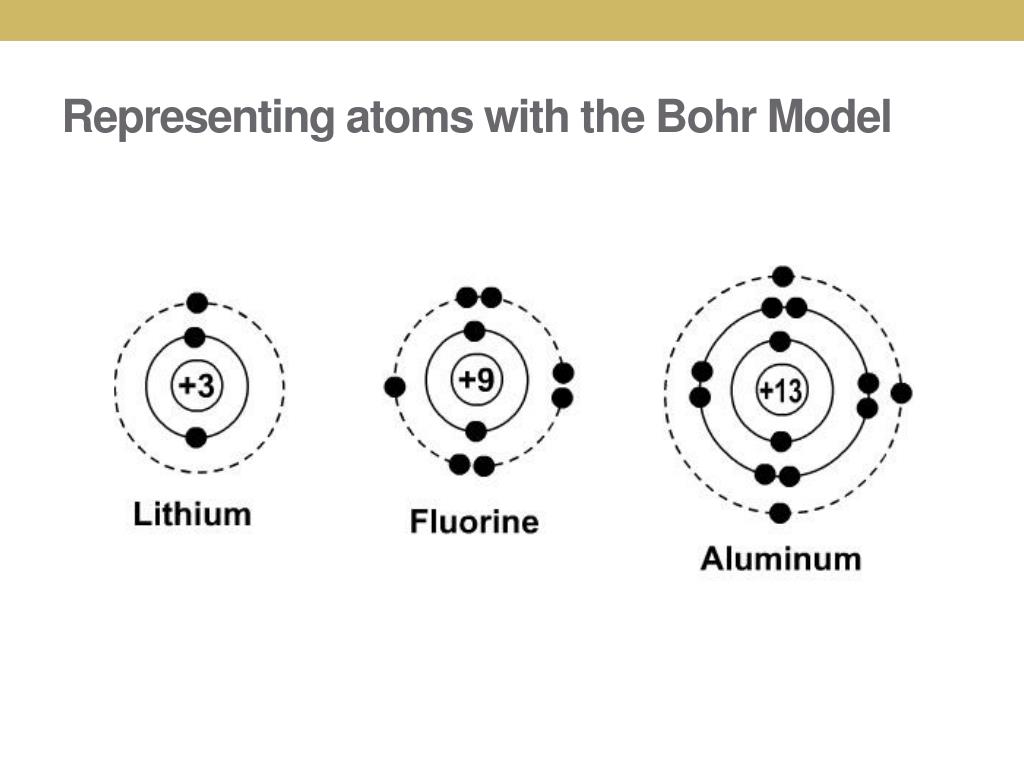
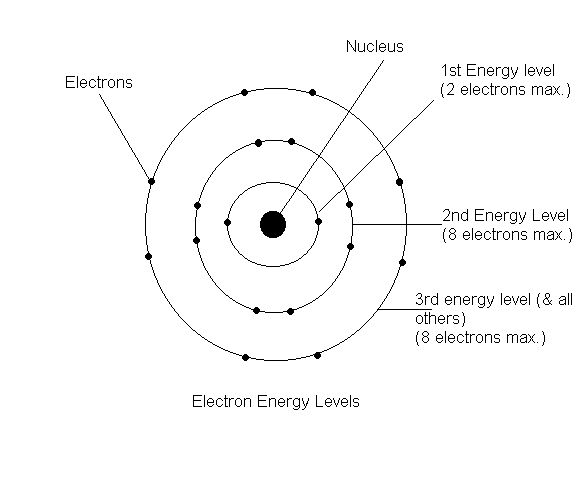
Aug 15, 2020 · Bohr Diagrams. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure \(\PageIndex{2}\) contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. draw a Bohr-Rutherford diagram for oxygen. draw a Bohr-Rutherford diagram for fluorine. a Bohr-Rutherford diagram is used to show the numbers and locations of protons, neutrons, and electrons in an atom. step 1. In atomic physics, the RutherfordBohr model or Bohr model or Bohr diagram, presented by Niels Bohr and Ernest Rutherford in , a system ...
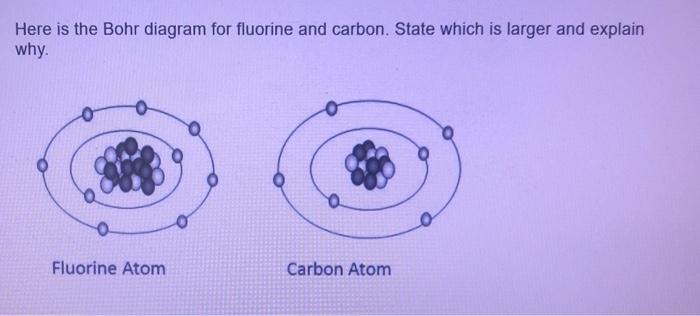
Fluorine has seven of eight possible electrons in its outermost energy level, which is energy level II. It would be more stable if it had one more electron because this would fill its outermost energy level. How do Bohr diagrams work? Bohr Diagrams. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around ...

Bohr diagram of fluorine
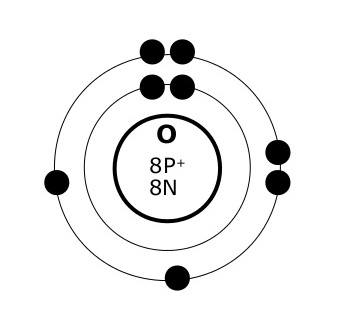
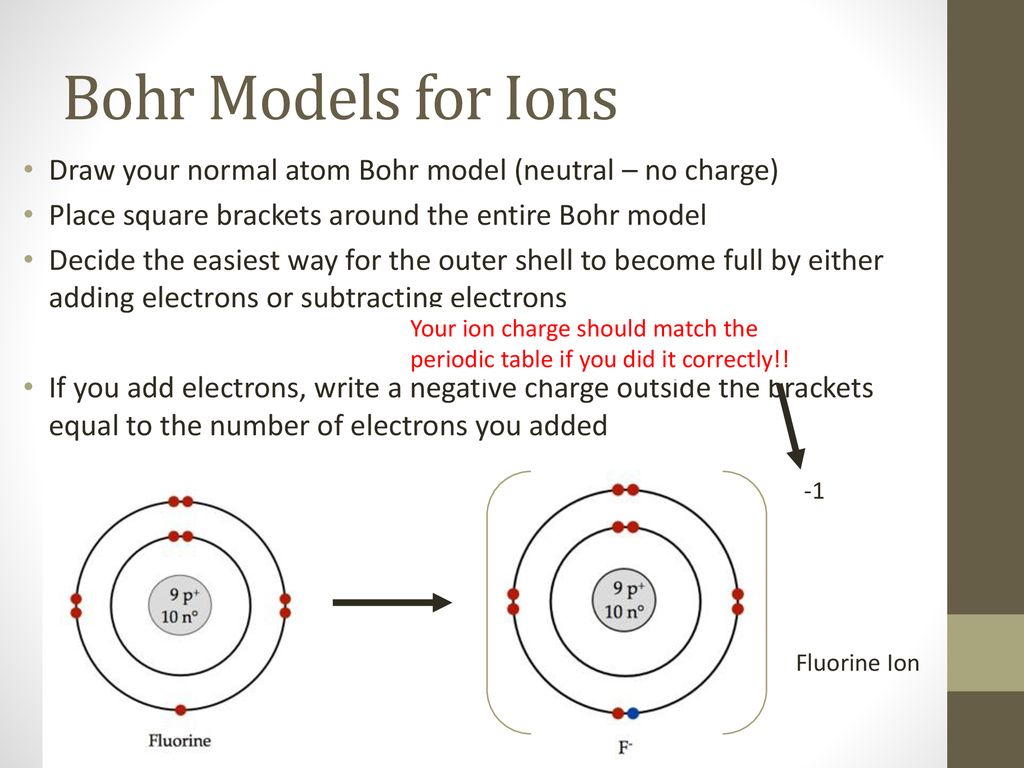
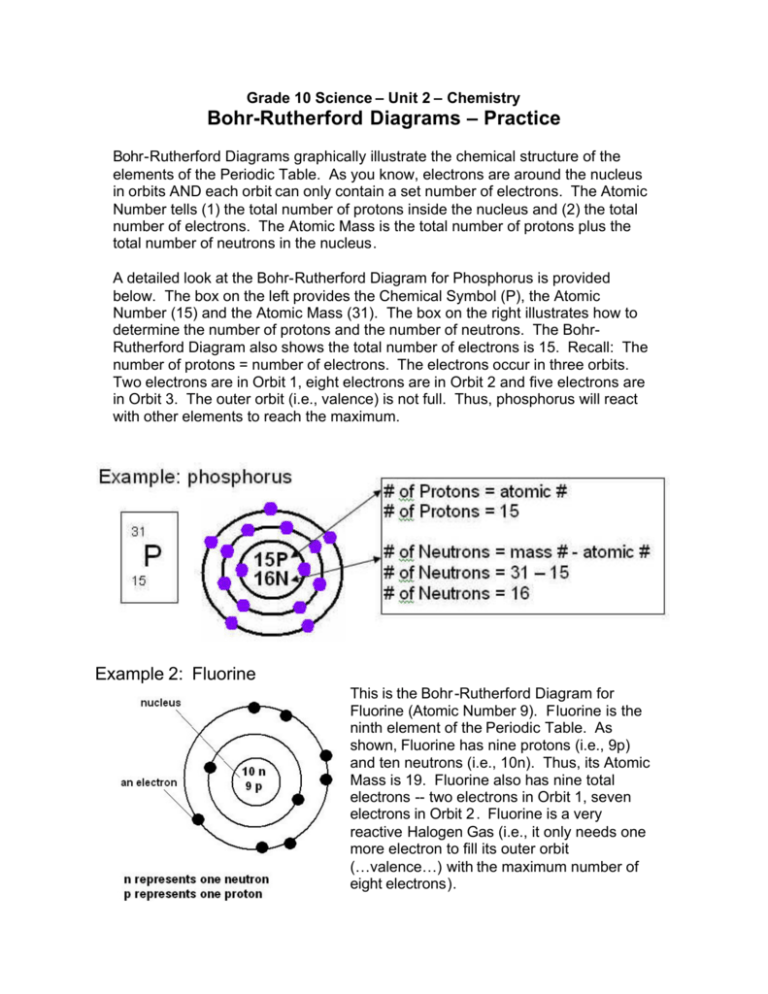
Nov 23, 2021 · The outermost shell in the Bohr diagram of Fluorine contains 7 electrons that also called valence electrons. The information gathered to make this model was all gathered from the periodic table. n0_____ Bohr Model of Fluorine Atom Now, using your Atomic Modeling materials, take an electron from sodium’s outermost energy level (shell) and give ... Draw a Bohr diagram of a fluorine atom. 2. Draw a Bohr diagram of a magnesium ion. Compounds using diagrams 1.notebook 2 March 20, 2019 _____: an atom that now has a positive or negative charge because it gained or lost a specific number of electrons. ... Bohr Diagram: The First Element. In order to make a Bohr diagram, you need to know the number of protons, neutrons, and electrons the element has. In this section, we'll show a sample Bohr diagram for hydrogen. H —Hydrogen. 1 proton. 1 electron. 0 neutrons
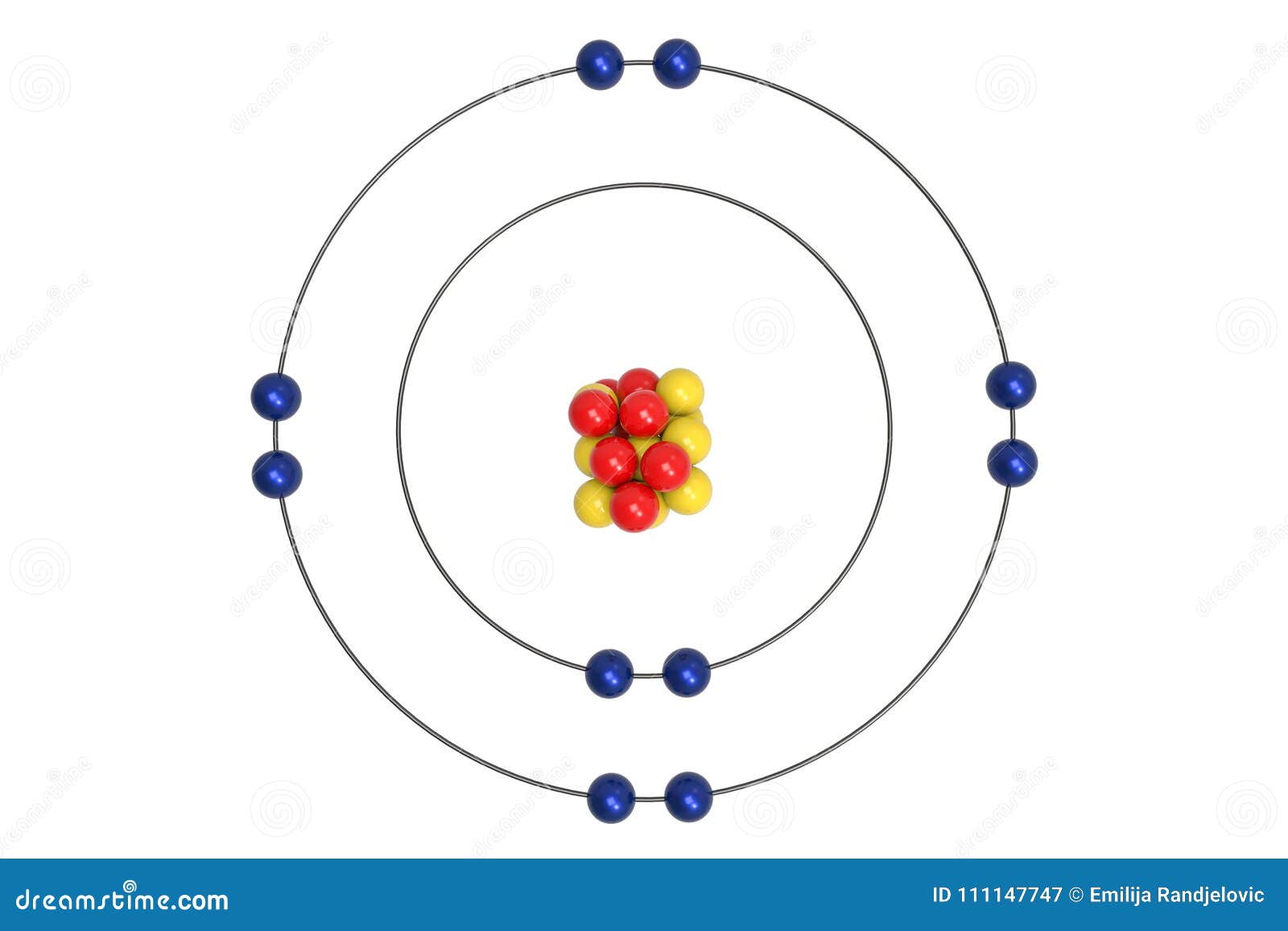
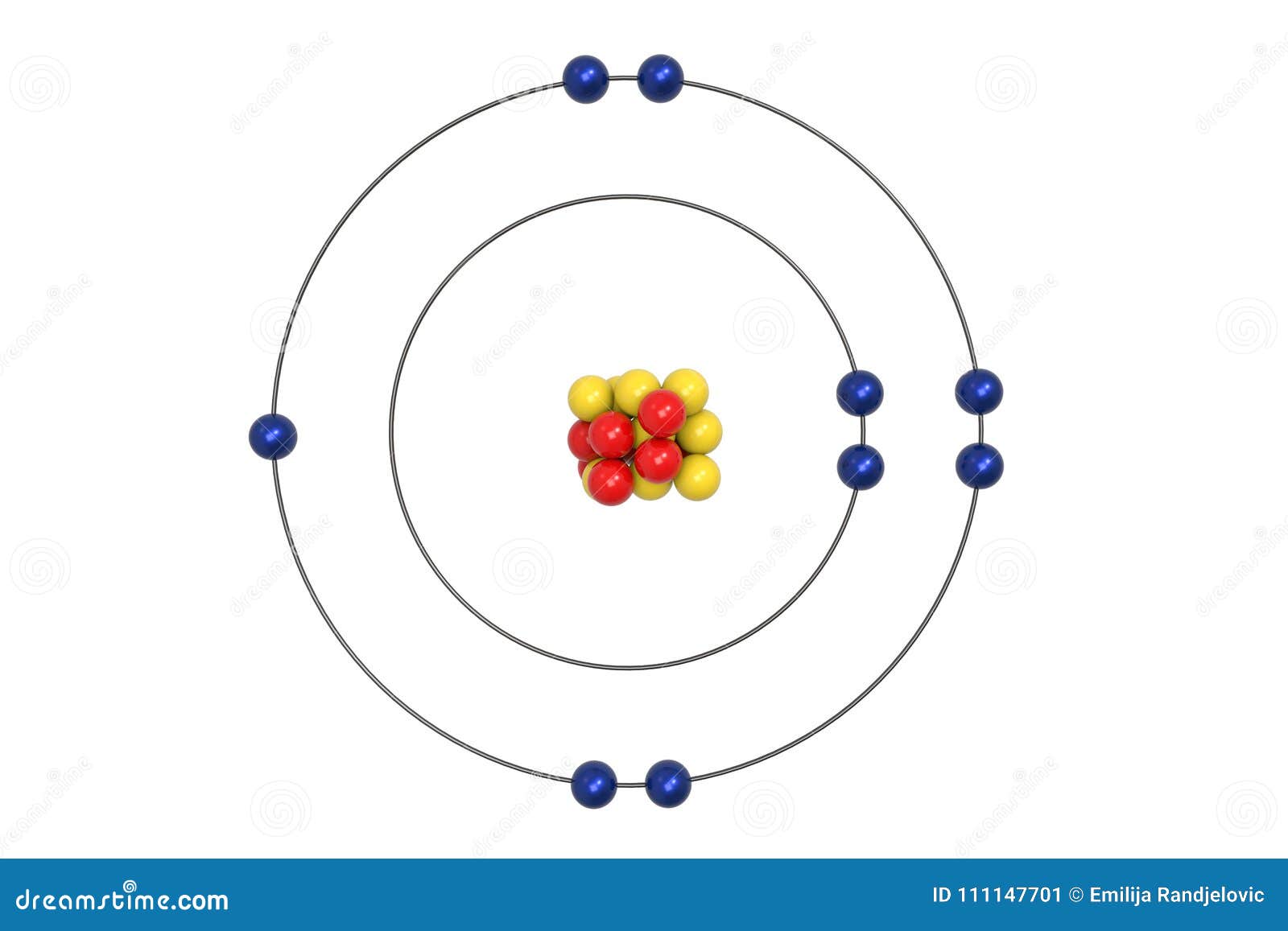
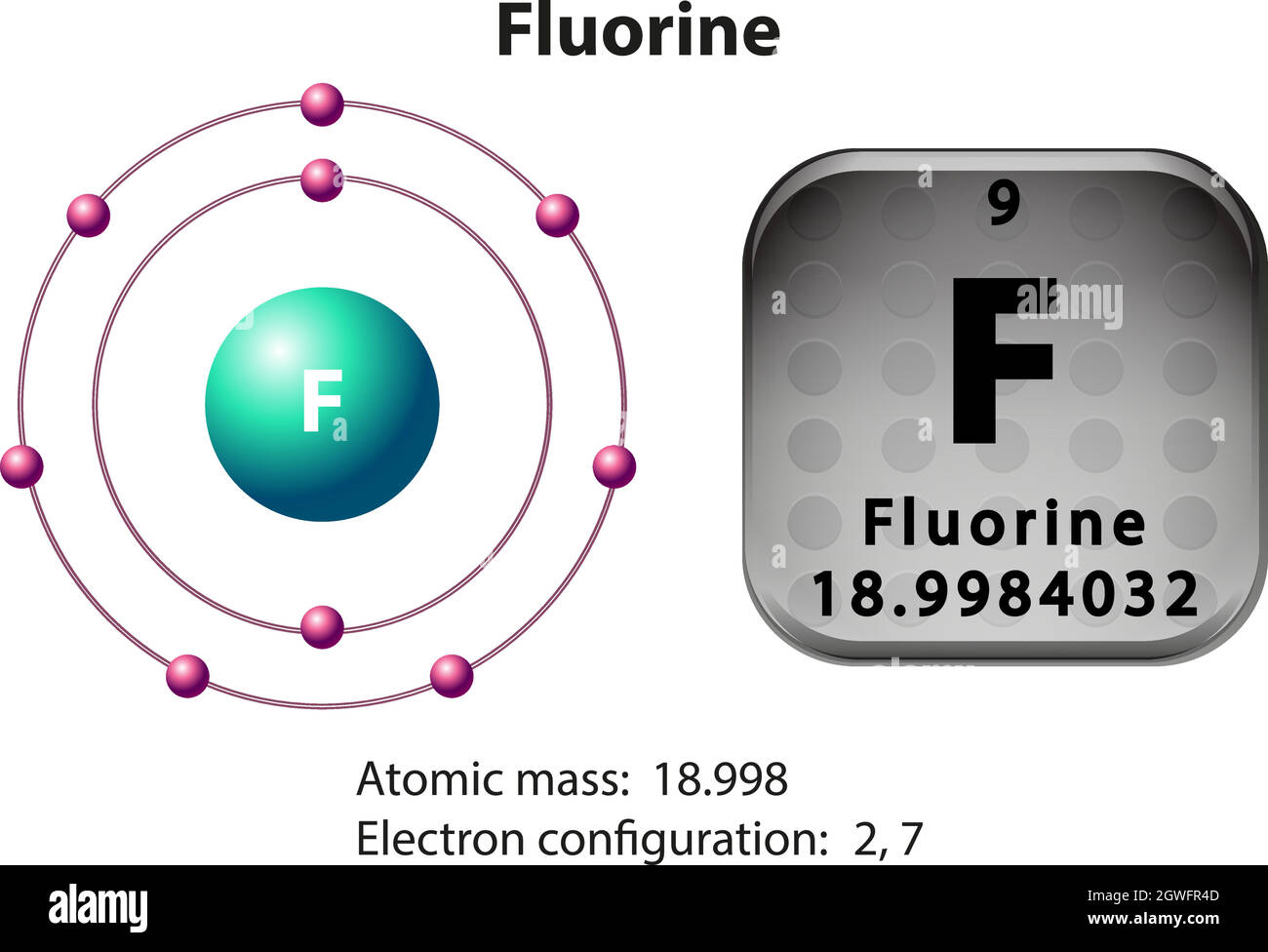
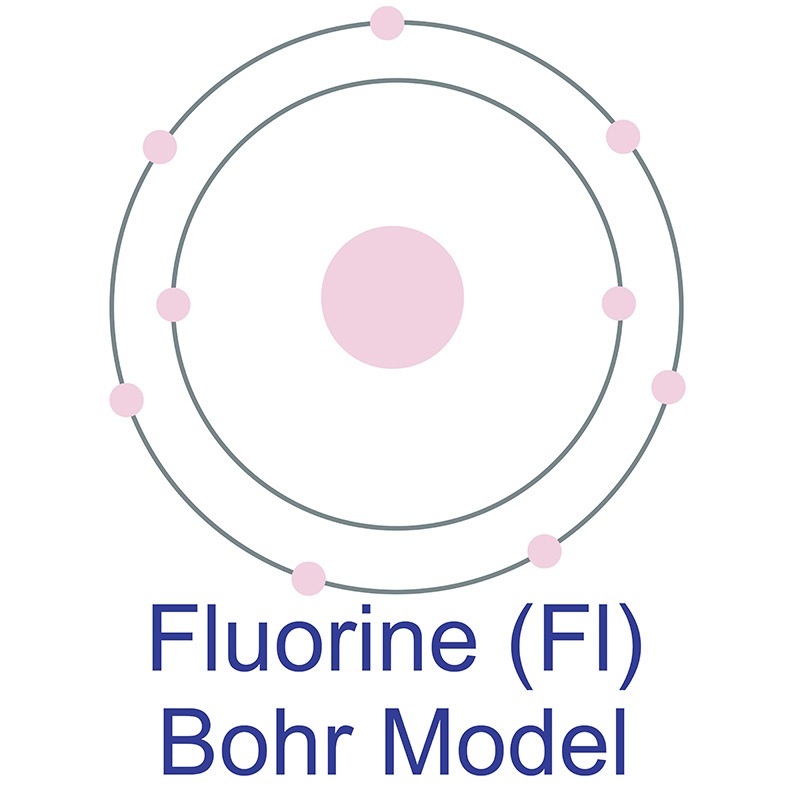
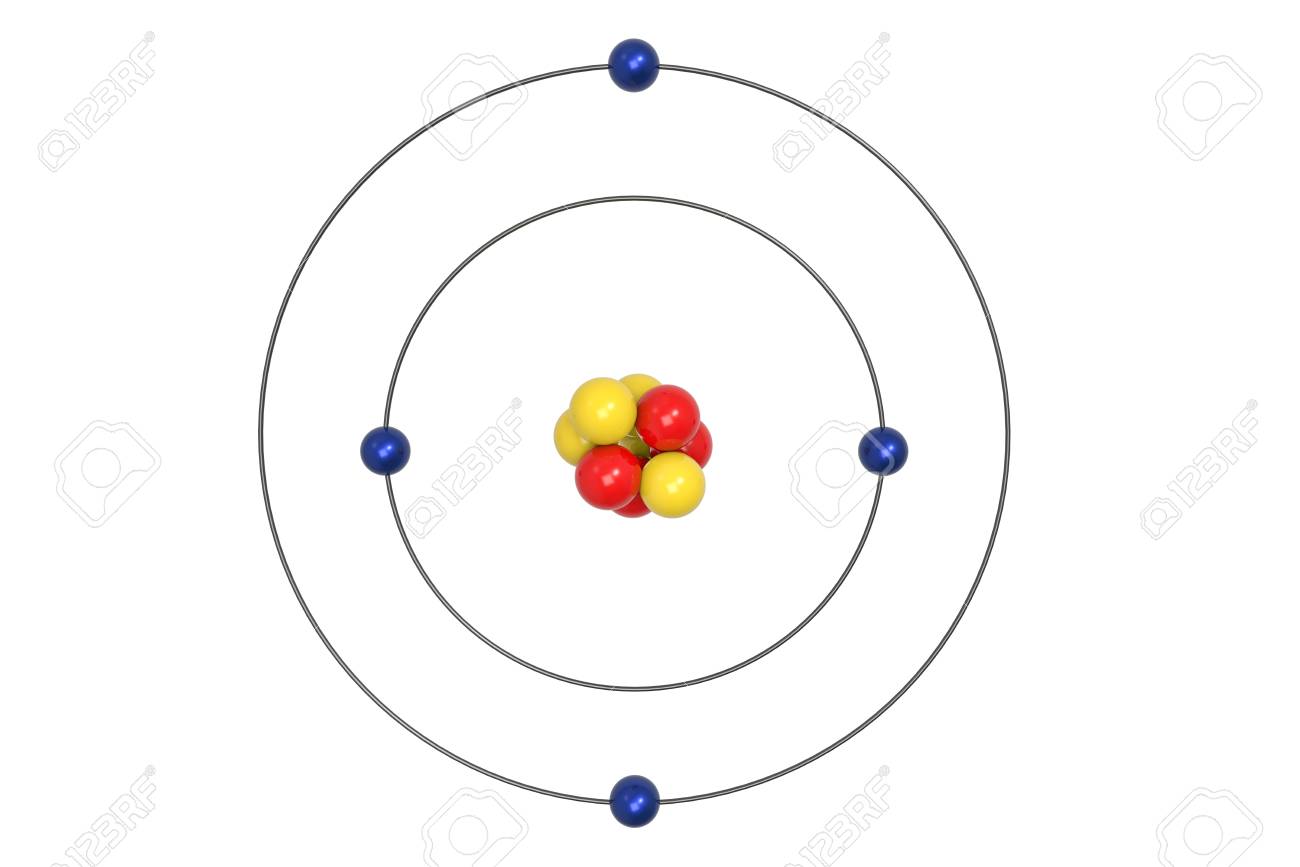
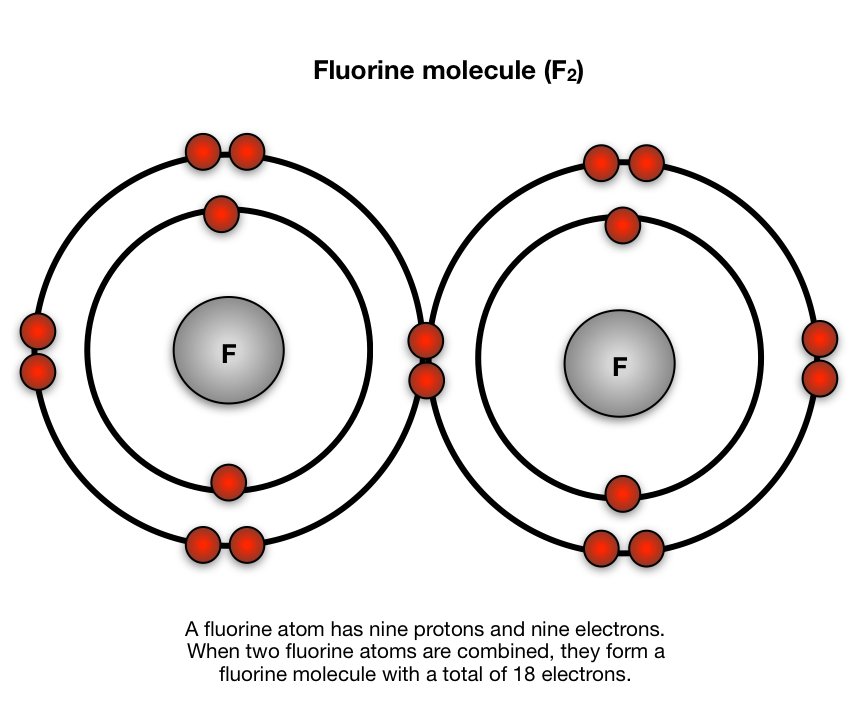
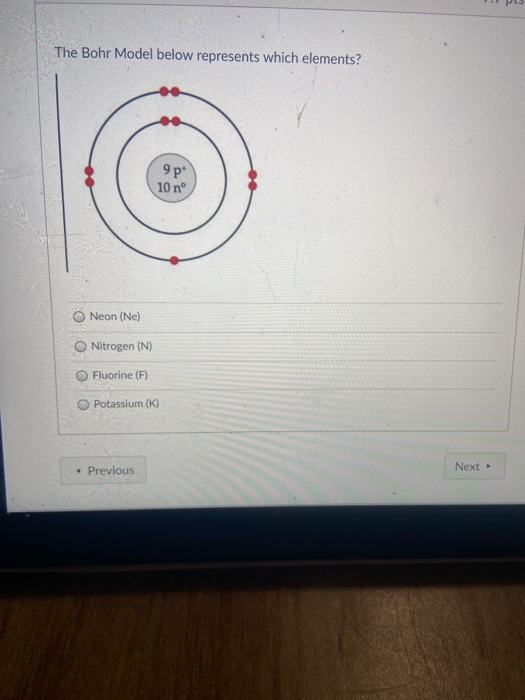
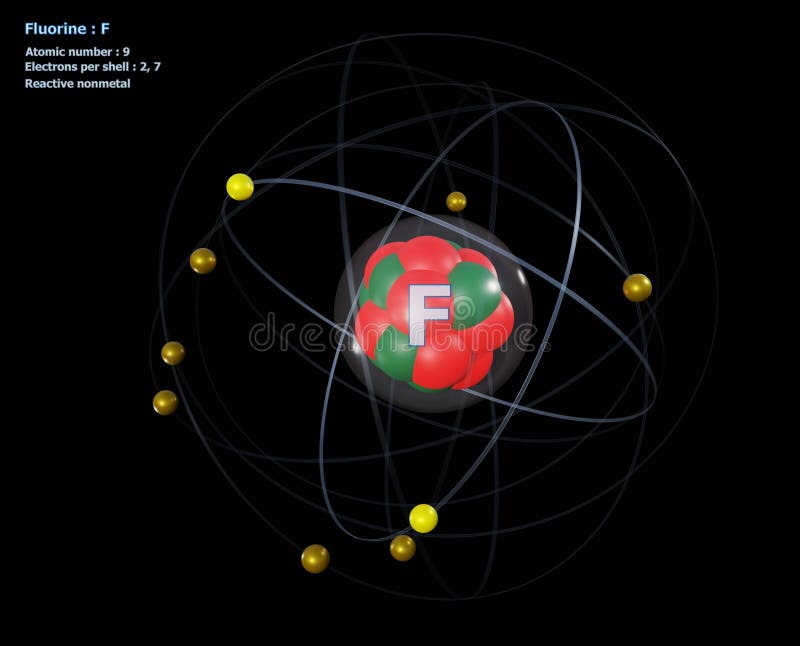
Bohr diagram of fluorine. The diagram below shows the Bohr model for fluorine. The nucleus of fluorine has 9 protons. Surrounding the nucleus of fluorine is 9 electrons. The electrons arrange themselves in 3 orbits: In the first orbit, there are 2 electrons. In the second orbit, there are 7 electrons. In the third orbit, there are no electron. According to Bohr's model of the atom, electrons orbit about the nucleus much like the way planets orbit the sun. Different energy levels are associated with the different orbits. The diagram below shows the Bohr model for fluorine. The nucleus of fluorine has 9 protons. Surrounding the nucleus of fluorine is 9 . Bohr diagram for fluorine. Fluorine has an atomic number of 9. This means there are 9 protons in the nucleus. Most fluorine around the world has 10 neutrons in the nucleus (mass number of 19 ... Photo "Bohr model of Fluorine Atom with proton, neutron and electron. Science and chemical concept 3d illustration" can be used for personal and commercial purposes according to the conditions of the purchased Royalty-free license. The image is available for download in high resolution quality up to 10000x6670.
Bohr Model of Fluorine Physical Science, Science Fair, Science And Nature, Atom Chlorine science model Atomic Structure Model, Atom Model Project, Bohr. Fluorine has an atomic number of 9. This means there are 9 protons in the nucleus. Most fluorine around the world has 10 neutrons in the nucleus (mass.Figure \(\PageIndex{2}\) contrast the Bohr ... The diagram below shows the Bohr model for fluorine. The nucleus of fluorine has 9 protons. Surrounding the nucleus of fluorine is 9 electrons. The electrons arrange themselves in 3 orbits: In the first orbit, there are 2 electrons. In the second orbit, there are 7 electrons. In the third orbit, there are no electron. Bohr deduced that: Fluorine has 2 electrons in its first shell and 7 in its second.Check me out: http://www.chemistnate.com Bohr model of Beryllium (Be) 2, 2: 5: Bohr model of Boron (B) 2, 3: 6: Bohr model of Carbon (C) 2, 4: 7: Bohr model of Nitrogen (N) 2, 5: 8: Bohr model of Oxygen (O) 2, 6: 9: Bohr model of Fluorine (F) 2, 7: 10: Bohr model of Neon (Ne) 2, 8: 11: Bohr model of Sodium (Na) 2, 8, 1: 12: Bohr model of Magnesium (Mg) 2, 8, 2: 13: Bohr model of ...
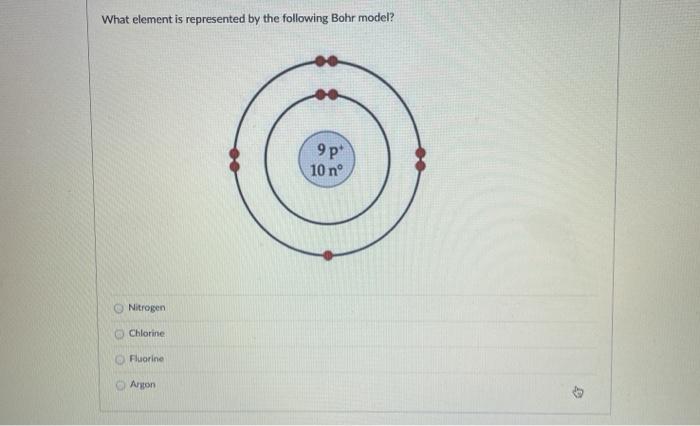
For more information and resources, please visit: https://sites.google.com/tdsb.on.ca/htc-meyer/home/9-Science/9-chemistry The Bohr Model of Fluorine(F) has a nucleus that contains 10 neutrons and 9 protons. This nucleus is surrounded by two-electron shells named K-shell and L-shell. The outermost shell in the Bohr diagram of Fluorine contains 7 electrons that also called valence electrons. 2. Use the table above to draw the Bohr model diagram for the following atoms and ions. Argon atom 22N Chlorine atom Chlorine ion 19 Potassium atom Potassium ion 3. What do you notice about the arrangement of electrons in the Bohr model of a neon atom, fluorine ion, and a magnesium ion? ALI e ec Levels 4. This video expectation is to understand how the Bohr-Rutherford was designed and effectively use a template to draw the Bohr-Rutherford diagram of the first ...
Lewis Dot Diagram Worksheet Use the Bohr models to determine the number of valance electrons. Once you have found the number of valance electrons, place them around the elements symbol. Element Atomic # Atomic Mass Protons Neutrons Electrons Lewis Dot Carbon 6 12 6 6 6 l Hydrogen 1 1 1 0 1 H Lithium 3 7 3 4 3 Li
This is a bohr model of fluorine. This is a nohr model of Iodine. This is a bohr model of Bromine. This entry was posted on February 20, 2007 at 6:08 pm and is filed under Uncategorized . You can follow any responses to this entry through the RSS 2.0 feed. You can leave a response, or trackback from your own site.
Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. 6p 6n. Bohr Diagrams Try the following elements one at a time: a) H b) He
Bohr Model Drawing Draw a Bohr model of a chlorine atom in the space below. Be sure to place the electrons in the correct orbitals and to fill out the key for the subatomic particles. Protons: Neutrons: Electrons: Chlorine 35.42 Atomic number equals the number of or Atomic mass equals the number of Identify the each of the parts of the box. Oxygen
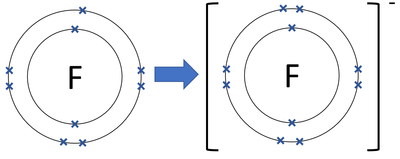
Diagrams - Public Service Announcement: Sodium Fluoride. Lewis Dot Diagram- The sodium transfers one electron to the fluorine so they can both become stable. Bohr-Rutherford Diagram: Sodium is the cation as it has a positive charge after losing one electron, while fluoride is the anion as it has a negative charge after gaining one electron.
Bohr Model Questions and Answers. Get help with your Bohr model homework. Access the answers to hundreds of Bohr model questions that are explained in a way that's easy for you to understand.
Bohr Diagram: The First Element. In order to make a Bohr diagram, you need to know the number of protons, neutrons, and electrons the element has. In this section, we'll show a sample Bohr diagram for hydrogen. H —Hydrogen. 1 proton. 1 electron. 0 neutrons
Draw a Bohr diagram of a fluorine atom. 2. Draw a Bohr diagram of a magnesium ion. Compounds using diagrams 1.notebook 2 March 20, 2019 _____: an atom that now has a positive or negative charge because it gained or lost a specific number of electrons. ...
Nov 23, 2021 · The outermost shell in the Bohr diagram of Fluorine contains 7 electrons that also called valence electrons. The information gathered to make this model was all gathered from the periodic table. n0_____ Bohr Model of Fluorine Atom Now, using your Atomic Modeling materials, take an electron from sodium’s outermost energy level (shell) and give ...
















0 Response to "40 bohr diagram of fluorine"
Post a Comment