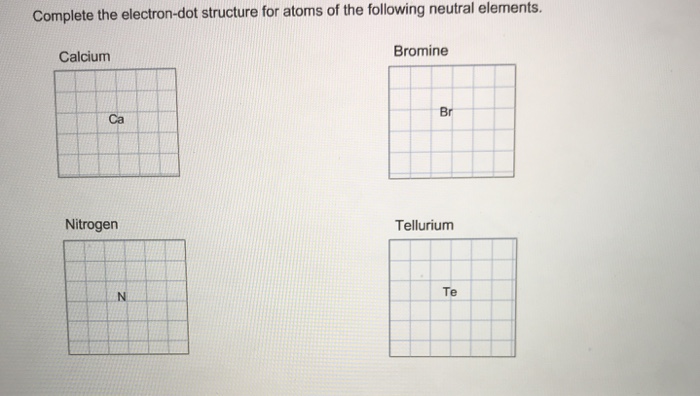
41 electron dot diagram for nitrogen
lewis structure n2 Nitrogen is a triple bonded molecule. Since Nitrogen belongs to the diatomic molecule in the VA family, on the periodic tables, which means that the valency of the molecule is five, therefore, it needs three more valences of electrons in order to complete its octet, and therefore, it is a triple bonded molecule. Lewis Structure of NO2. A molecule of nitrogen dioxide consists of one nitrogen atom and two atoms of oxygen. Let us look at the periodic table. Nitrogen belongs to group 15 ( or group 5) and has an atomic number of 7, therefore has a valency of 5. Oxygen belongs to group 16 ( or group 6) and has an atomic number of 8, therefore a valency of 6.
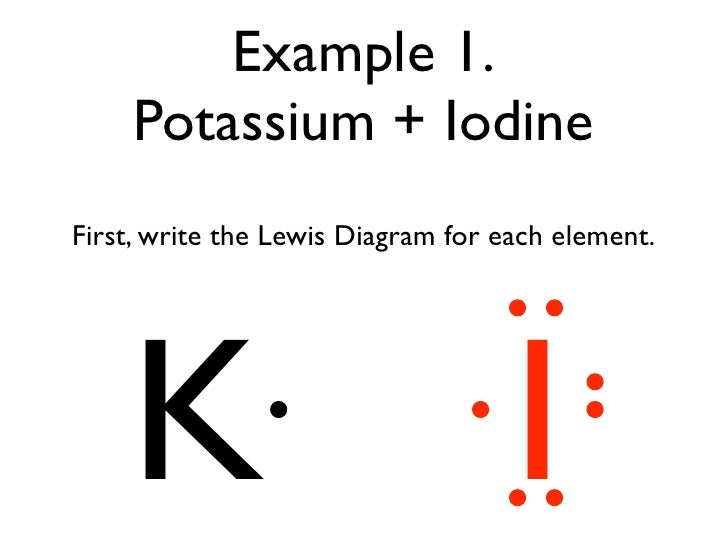
What is the Lewis dot structure for MG? Magnesium is element #12. To draw the Lewis electron dot diagram we picture in our minds the symbol for Mg in a box with all of its core electrons (i.e., 1s22s22p6). Then we place the valence electrons around the sides of the box with each side representing an orbital in the outermost energy level.
/NO2_Dot-56a12a2c3df78cf772680359.png)
Electron dot diagram for nitrogen
14+ Electron Dot Structure Of Nh3. Alternatively a dot method can be used to draw the nh3 lewis structure. We're going to do the lewis structure for nh3: NH3 Lewis and 3-D Structure- Dr. Sundin - UW-Platteville from people.uwplatt.edu Nitrogen has 5 valence electrons, but notice that nh4+ is a… The Lewis structure of XeF2 shows two bonding pairs and three lone pairs of electrons around the Xe atom: XeF6: We place three lone pairs of electrons around each F atom, accounting for 36 electrons. Use covalent Lewis structure to explain why the compound that forms between nitrogen and hydrogen has the formula NH_3. Below is the electron dot structure for a Nitrogen molecule: In the routine Table, Nitrogen is inserted in team 5 across duration 2. Thus, together per the electronic configuration the the element i.e. 2,5, the has five electrons in its outermost valence shell.As every the molecule N2, it has two atoms of Nitrogen.
Electron dot diagram for nitrogen. You see, there are 4 hydrogen atoms approximately nitrogen atom. Therefore, nitrogen atom is the facility atom. Also, over there is a +1 charge on nitrogen atom. Steps of drawing lewis framework of NH4+ There are several actions to draw the lewis structure of NH4+. But, These actions are described in detail in this tutorial. Draw electron dot structure for ammonia molecule. The ammonia molecule consists of 3 hydrogen atoms that are covalently bound to one nitrogen atom. Calculate the total valence electrons in NH3 molecule. NH4 Lewis Structure - How to Draw the Dot Structure for NH4 Ammonium Ion A step-by-step explanation of how to write the Lewis Dot Structure for ... What is the Lewis dot diagram for nitrogen? Each N is surrounded by two dots and three sticks or lines, representing another 6 electrons in the N2 triple bond. So each N is surrounded by 8 total valence electrons, giving it an octet and making it stable. The two letter N's in the N2 Lewis structure represent the nuclei (centers) of the ... To get the stable octet electronic configuration nitrogen acquire three electrons and three chlorine atom share one electron to the nitrogen atom. 14+ Electron Dot Structure Of H2S. Sometimes we use an x instead of. Approach requires one to define an effective one electron hamiltonian, hˆeff.
The electron dot diagram for NOF is as follows: The N atom has three electron groups on it, two of which are bonded to other atoms. The molecular shape is bent.. What is the Lewis structure for Nof?, The NOF Lewis structure is very similar to NOCl and NOBr. In the NOF Lewis structure Nitrogen (N) is the least electronegative atom and goes in the center of the Lewis structure. Drawing the Lewis structure for CH 4 (named methane) requires only single schematron.org's one of the easier Lewis structures to draw. draw electron dot structure of ethane and butane, Figure 4 demonstrates drawing Lewis electron dot structures for these compounds. give the a electron dot diagram of i magnesium chloride ii nitrogen iii methane ... Headache, woke up later than usual. Only Chem P1 tips today. To those who are currently taking their papers, good luck with them. ​ Second last day, time sure fly fast. Wash up, have a good breakfast, drink some water, whatever it takes to not burn out. Go run an hour before the exam if you wish to, just don't be late. If you forgot your calculator, either borrow from others or learn quick maths. ​ Papers today: \- (1153/1151/1152)/01 Chinese/Malay/Tamil B P1 08:00 - ... Note: I take Pure Chem + Bio. All Physics tips are courtesy of my classmates and this community ([https://www.reddit.com/r/SGExams/comments/qqr4pn/o\_levels\_pure\_physicscombined\_science\_p1\_tips/](https://www.reddit.com/r/SGExams/comments/qqr4pn/o_levels_pure_physicscombined_science_p1_tips/)) ​ 3rd last exam day till the end. Might be your last day for some. Either way, hope you had a good rest yesterday. Pure Physics starts at 8am, so make sure to head to school earlier. &a...
What is the correct Lewis structure for nitrogen?, Each N is surrounded by two dots and three sticks or lines, representing another 6 electrons in the N2 triple bond. So each N is surrounded by 8 total valence electrons, giving it an octet and making it stable. The two letter N's in the N2 Lewis ... The atoms are as follows: The two atoms can share their unpaired electron: Exercise 7.4. 1. Use Lewis electron dot diagrams to illustrate the covalent bond formation in Cl 2. Answer. More than two atoms can participate in covalent bonding, although any given covalent bond will be between two atoms only. HCN Lewis Structure, Molecular Geometry, Shape, and Polarity. Hydrogen Cyanide is a colorless, flammable, and poisonous chemical liquid. Represented by the chemical formula, HCN is one of those molecules that has an interesting Lewis structure. This liquid is used in electroplating, mining, and as a precursor for several compounds. N2H2 Lewis structure, Molecular Geometry, Hybridization, Bond Angle and Shape. N2H2 is a chemical formula for a Diazene molecule which is also known as Nitrogen Hydride. It is the conjugate acid of a diazenide. The molecule is made up of two hydrogen atoms and two nitrogen atoms.
electron dot diagram for oxygen › lewis dot diagram for oxygen 2 › lewis dot ... Although neither arrangement will immediately fulfill the conditions that oxygen has two bonds and nitrogen has three Lewis diagrams are electron dot pictures which give an excellent account of the number of valence electrons in a covalent molecule.
hi guys imma just type the summary of things we need to know for chem o lvl's tmr (pls add on in the comments if i miss out something so i can edit this again) in hoping to help out others while also helping me to revise bcos typing notes is wayy faster than writing :) also this wld not be super precise bcos im just typing in the summary if not i'm literally typing a whole textbook here, but i'll try my best to put in all the infos that is important \*totally not last minute\* **kinetic partic...
The Lewis structure of N2h2 is an example of a molecule that can be described by resonance. This means that the nitrogen atom has 6 valence electrons, but it only shares 4 electron pairs with hydrogen atoms. The remaining two are shared between the two nitrogen atoms in order to give each atom 8 valence electrons.
NF3 lewis structure has 3 fluorine and 1 nitrogen atom connected with three single bonds. This structure is very similar to NCl3 and NH3. There is a total of 10 lone pairs and 3 bonded pairs present in the NF3 lewis structure.
[\[](https://cdn.discordapp.com/attachments/727580829924196413/810525294909849600/Silver_Purple_ThickPNG.png)[Disclaimer\]](https://www.reddit.com/user/Zephylandantus/comments/ljpssb/disclaimer/) [\[First\]](https://www.reddit.com/r/HFY/comments/j66pee/tev_tricard/?utm_medium=android_app&utm_source=share) [\[Wiki\]](https://www.reddit.com/r/HFY/wiki/series/tev_tricard) [\[Previous\]](https://www.reddit.com/r/HFY/comments/p88yqy/tev_tricard_home/) ​ Hansen stood on the bridge a...
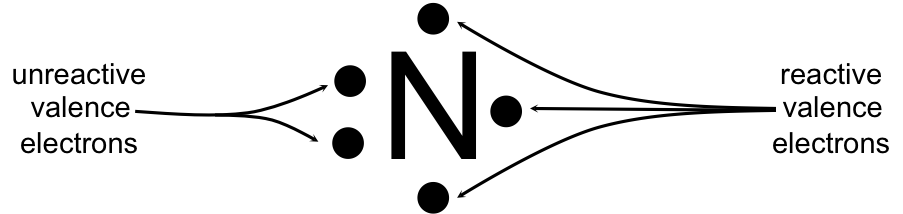
For an ion, the electron dot structure shows the number of valence electrons in the ion.. The Lewis dot structure shows valence electrons as dots.These valence electrons are the electrons in the outermost shell of an atom or ion. They are the electrons that may participate in a chemical reaction. The electron dot structures of nitrogen, oxygen, and fluorine ions are shown in the images attached.
Note: I take Pure Chem. Heard Combi Chem marking scheme's more lenient. Still, make your own judgement when reading the tips. ​ Today's Tuesday. And again, wash up, eat breakfast, drink water, bring what you need, and prepare yourself for today. Hope yesterday's paper didn't stress you out too much if you took it. ​ Papers today: \- 5076/03 Science (Phy/Chem) P3 (Chem) 14:30-15:45 \- 5078/03 Science (Chem/Bio) P3 (Chem) 14:30-15:45 \- 6092/02 Chem P2 14:30-1...
Steps to Draw the Lewis structure of N2. Below is the electron dot structure for a Nitrogen molecule: In the Periodic Table, Nitrogen is placed in Group 5 across Period 2. Thus, as per the electronic configuration of the element i.e. 2,5, it has five electrons in its outermost valence shell. As per the molecule N2, it has two atoms of Nitrogen.
The structure on the right is the Lewis electron structure, or Lewis structure, for H 2 O. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. Moreover, by sharing a bonding pair with oxygen, each hydrogen atom now has a full valence shell of two electrons.
Chemistry Lewis Structure. Qno 3: Lewis structure nof. Ans: In the Nof Lewis structure, Nitrogen (N) is the least electronegative atom and is the center of the whole lewis structure. In the lewis structure there are totally 18 valence electrons. Qno 4: Lewis structure for nof.
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom.
What is the Lewis structure for the ammonia molecule? Since nitrogen belongs to the group VA, it has five valence electrons, and then: D = 1 × 5 (one nitrogen atom) + 1 × 3 (three hydrogen atoms) = 8 electrons. N = 8 × 1 + 2 × 3 = 14 electrons. C = 14 - 8 = 6 electrons. C / 2 = 3 links
Steps to Draw the Lewis structure of N2. Below is the electron dot structure for a Nitrogen molecule: In the Periodic Table, Nitrogen is placed in Group 5 across Period 2. Thus, as per the electronic configuration of the element i.e. 2,5, it has five electrons in its outermost valence shell.As per the molecule N2, it has two atoms of Nitrogen.
Below is the electron dot structure for a Nitrogen molecule: In the routine Table, Nitrogen is inserted in team 5 across duration 2. Thus, together per the electronic configuration the the element i.e. 2,5, the has five electrons in its outermost valence shell.As every the molecule N2, it has two atoms of Nitrogen.
The Lewis structure of XeF2 shows two bonding pairs and three lone pairs of electrons around the Xe atom: XeF6: We place three lone pairs of electrons around each F atom, accounting for 36 electrons. Use covalent Lewis structure to explain why the compound that forms between nitrogen and hydrogen has the formula NH_3.
14+ Electron Dot Structure Of Nh3. Alternatively a dot method can be used to draw the nh3 lewis structure. We're going to do the lewis structure for nh3: NH3 Lewis and 3-D Structure- Dr. Sundin - UW-Platteville from people.uwplatt.edu Nitrogen has 5 valence electrons, but notice that nh4+ is a…
Using electron-dot diagrams which show only the outermost shell electrons show how a molecule of nitrogen N2 is formed from two nitrogen atoms. - Studyrankersonline
Unit 3 ATOMIC CONCEPTS 3.9 What is a Lewis Diagram AIM: How do I draw a Lewis Diagram of any element? DO NOW: Draw a planetary model of Oxygen. How many. - ppt download
The three lines in this Lewis dot structure represent the. A) three valence electrons of dinitrous - Brainly.com





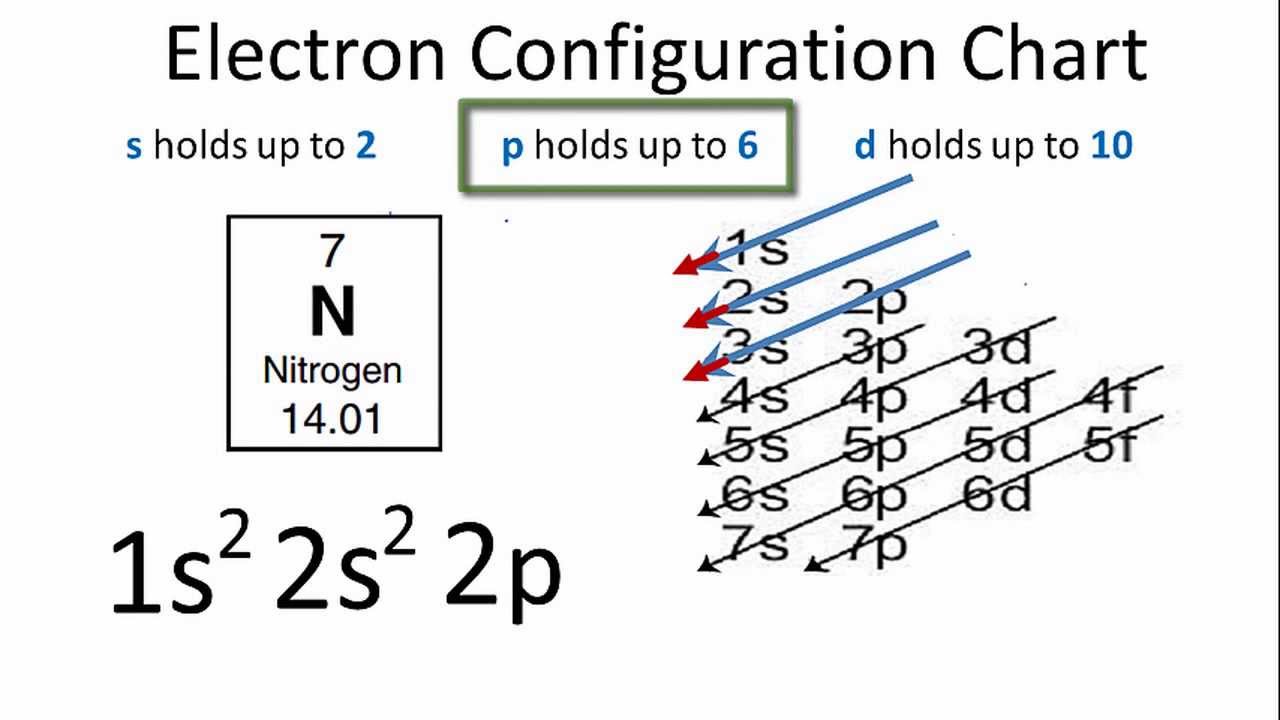

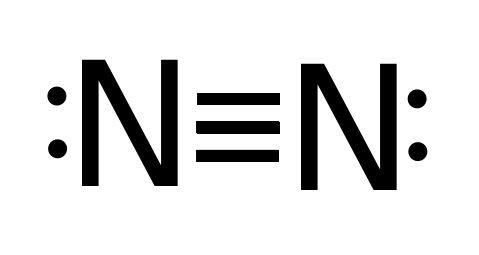















0 Response to "41 electron dot diagram for nitrogen"
Post a Comment