41 lewis diagram for ch4
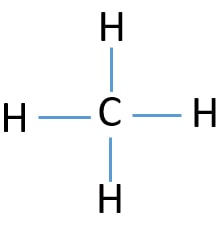
Methane is a one-carbon compound in which the carbon is attached by single bonds to four hydrogen atoms.It is a colourless, odourless, non-toxic but flammable gas (b.p. -161℃). It has a role as a fossil fuel, a member of greenhouse gas and a bacterial metabolite. Diagram 1, because all bond angles are 180°. Diagram 1, because all atoms have a formal charge of 0. Diagram 2, because the molecule has a trigonal pyramidal shape. Diagram 2, because all atoms have a formal charge of 0.
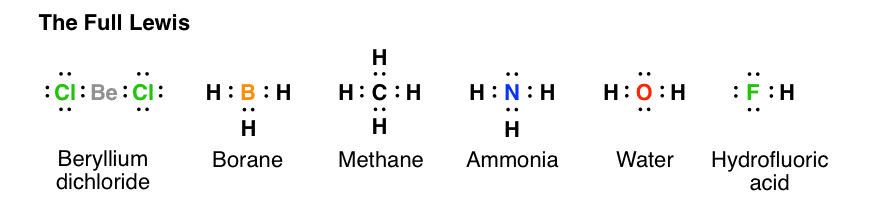
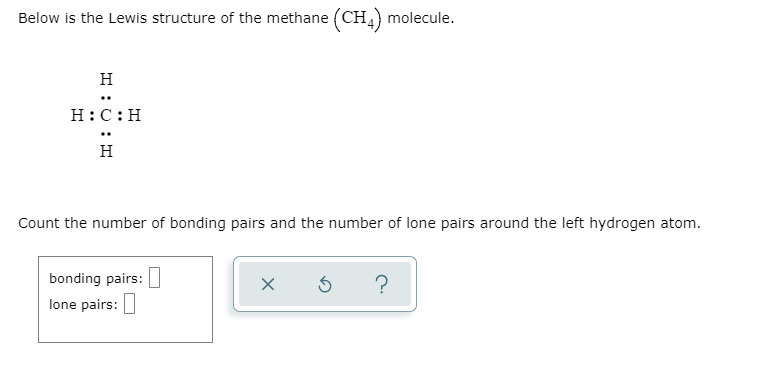
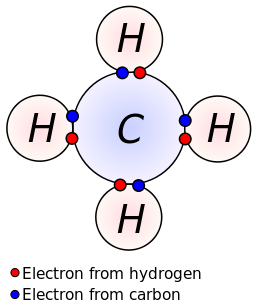
Methane (CH 4) Molecule Lewis Structure. Methane lewis structure contains four C-H bonds. There are no lone pairs in the valence shells of carbon atom. Carbon atom is the center atom and it is very easy to draw CH4 lewis structure. CH 4 lewis structure. There are following specifications in the lewis structure of methane.

Lewis diagram for ch4
Answer: I would be lazy and look it up on the internet. But seriously, you have an electron pair between the C and each of the H's in the Lewis diagram a ala Why is that the correct diagram, you ask? First, each Hydrogen has only one electron to donate or share, and remember that Hydrogen's fil... In addition. Lewis Structures for CH4. Step-by-step tutorial for drawing the Lewis Structure for CH4. Lewis Dot Structure for CH4 #2 Find the number of "octet" electrons for the molecule. C: 8 octet electrons x 1 atom = 8 octet electrons H: 2 octet electrons x 4 . We show two ways to draw the CH4 Lewis structure, methane. How to Draw the Lewis Dot Structure for CH4: MethaneA step-by-step explanation of how to draw the CH4 Lewis Dot Structure (Methane).For the CH4 structure use...
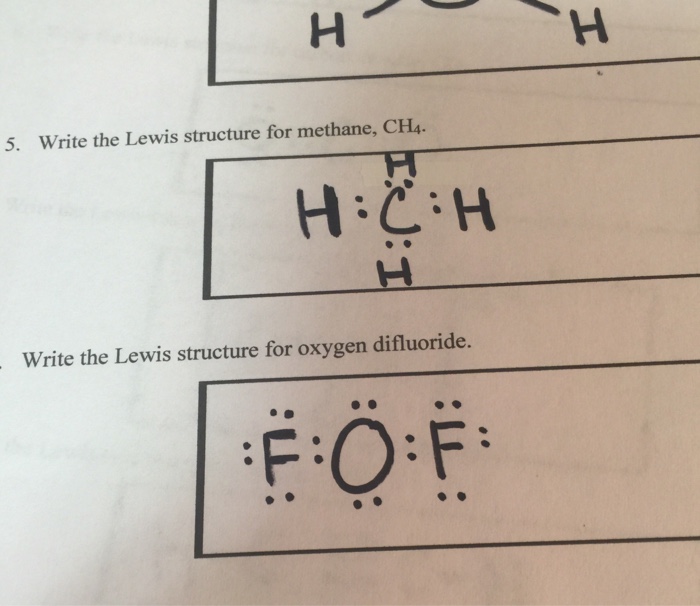
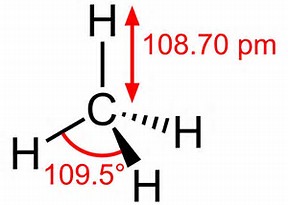
Lewis diagram for ch4. The lewis structure of CH4 is drawn to fulfill the need of valence electron by every the atoms. Lewis structure of CH4. The lewis structure of carbon and hydrogen atom says- to form a solitary CH4 molecule, a complete of eight valence electrons take part in the common bonding to fulfill the require of eight an ext valence electrons. CH4, commonly known as methane, is a tetrahedral structure with four hydrogen atoms forming around a central carbon atom . Pictorially, this structure resembles a pyramid in shape, with all four corners equidistant from the center. 15 Ch4O Lewis Structure. Remember that hydrogen atoms always go on the outside of a lewis structure and that they only need two valence electrons for a full outer shell. The xe atom is attached to all four oxygen atoms. Worksheet 1 - solutions A - CHEM 2400 Video 1-2… What is the correct Lewis dot diagram for CH4? Next Worksheet. Print Lewis Structures: Single, Double & Triple Bonds Worksheet 1. How do you represent a triple bond in a structural formula? ...
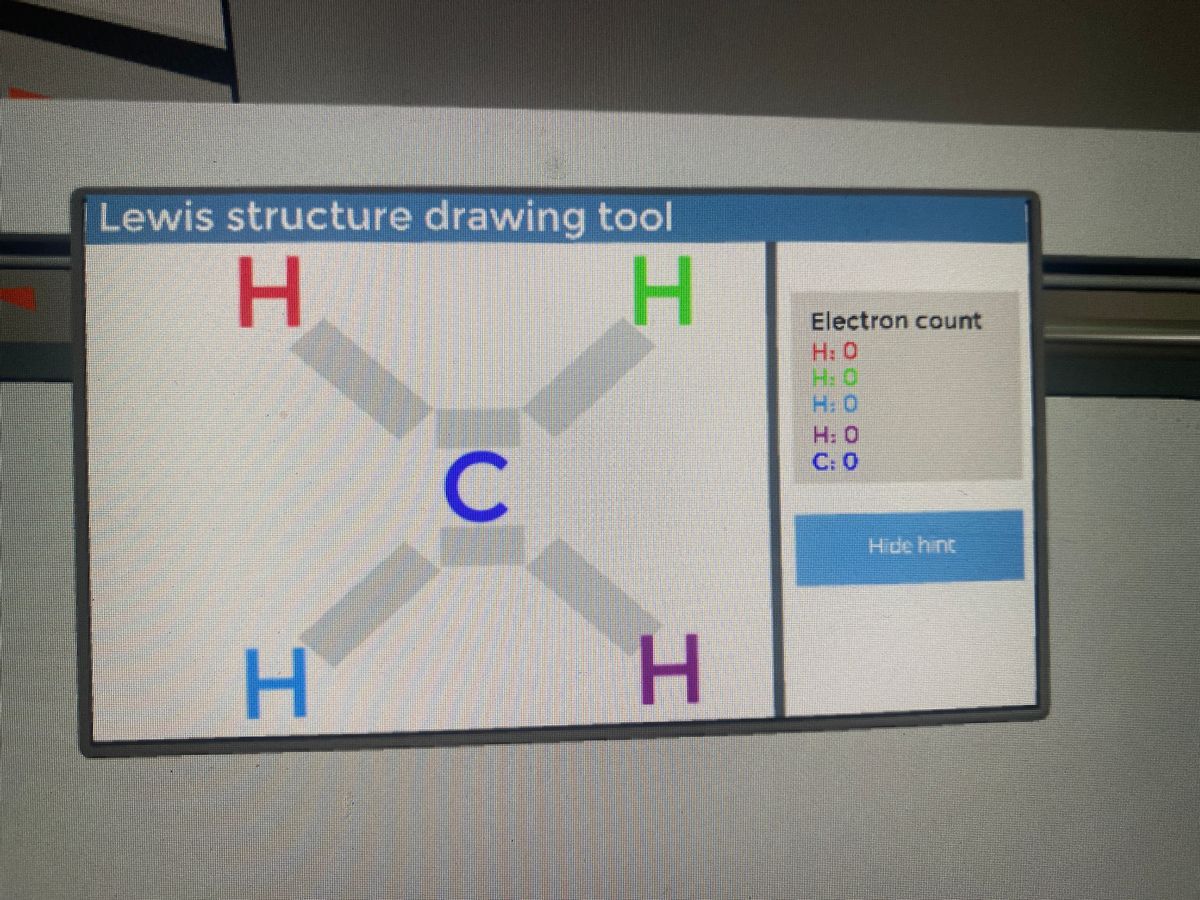
In this article, we will study the Cyanide (CN-) lewis structure, molecular orbital diagram(MO), its bond order, formal charges, and hybridization. Cyanide can be a colorless gas in the form of hydrogen cyanide, sodium cyanide, potassium cyanide, etc. The CH4 Lewis structure is a diagram that illustrates the number of valence electrons and bond electron pairs in the CH4 molecule. The geometry of the CH4 molecule can then be predicted using the Valence Shell Electron Pair Repulsion Theory (VSEPR Theory), which states that molecules will choose a CH4 geometrical shape in which the electrons ... Lewis Structures for CH4. Step-by-step tutorial for drawing the Lewis Structure for CH4. The Lewis Dot Structure for CH4 is shown above. These kinds of structures can also be shown by representing each of the bonds with two dots. Each atom in the . I will explain this with pictures, and some captions. This is just the five atoms in CH4, or Methane. Get the free "Lewis structure" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.
methane lewis structure dot ch4 structures following ch study derived steps writing general . ch4 lewis dot diagram structure draw structures methane electron bonds shown definition ch created . diagram ch4 dot covalent bond lewis structure electron label methane dash single form bins energy labeled atom conversion carbon brainly ... Answer: B carbon. Explanation. Lewis structure or dot structure is an easy way to get the bonding details of atoms in a molecule. If we talk about methane molecule carbon is central atom with four electrons that are bonded to four hydrogen atoms and each bond is single covalent bond.. Please see attached figure, Lewis structure of ch4. The compound is one of the. A covalent molecule contains at least two atoms sharing some number of valence electron pairs through one or more covalent bonds. The physical properties of the molecule like boiling point surface tension etc. Remember that uncharged carbon has 4 bonds and no lone pairs and hydrogen has one ... Procedure for Constructing Molecular Orbital Diagrams Based on Hybrid Orbitals. 1. Begin with the Lewis Molecular Orbital of Methane, CH4. 1. The Lewis. Molecular Orbital theory (MO) is the most important quantum mechanical theory This particular diagram shows the orbitals for both the hydrogen atom and the.
CH4 Lewis Structure. Answer: CH4 Lewis structure (Methane electron dot structure) is that type of diagram where we show the total 8 valence electrons of CH4 as dots , or dots and dashes (-).In Lewis structure,it is common that a bonding pair of two electrons can be shown by dash (-) or dots ( ) but a lone pair of two electrons is shown by dots [ ].
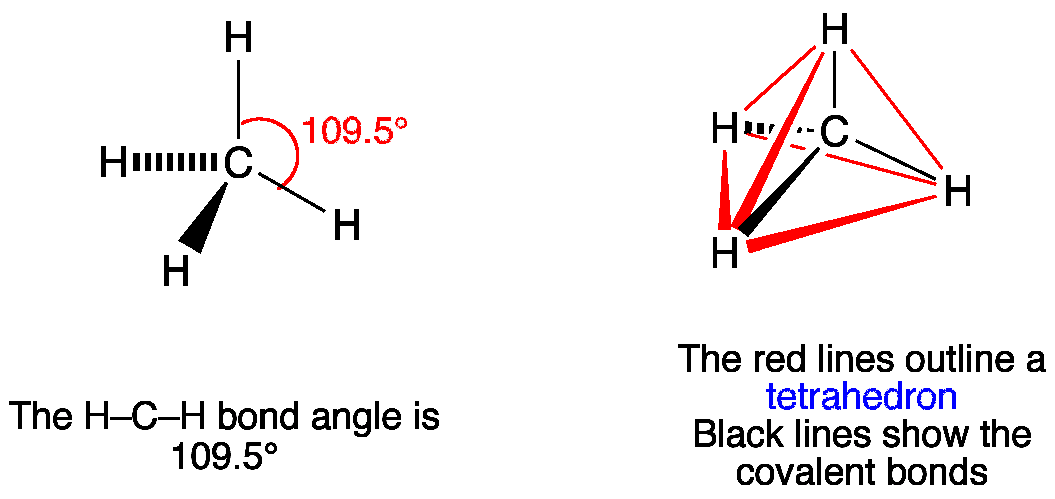
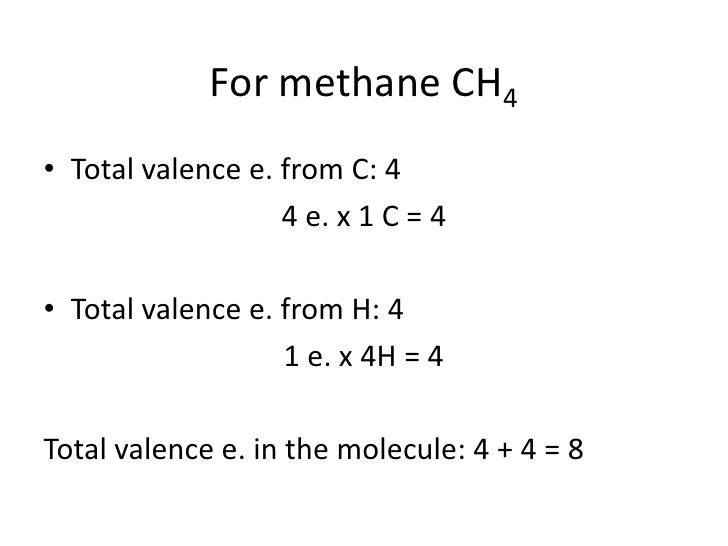
The total valence electron available for drawing the Methane (CH4) lewis structure is 8. The steric number of the central atoms in methane is 4 that ensures that it has an Sp 3 hybridization. CH4 is a nonpolar molecule due to its symmetrical geometry that causes uniform charge distribution all over the atom leads to a zero net dipole moment and ...
NO DOTS Draw the e- dot diagram for the – ion COMPLETE outer shell Step 3 Enclose both in brackets and show each charge Draw the Lewis Diagrams LiF MgO CaCl2 K2S Drawing molecules using Lewis Dot Structures Symbol represents the KERNEL of the atom (nucleus and inner e-) dots represent valence e- Always remember atoms are trying to complete ...
CH4 Lewis Structure Lewis structure is the pictorial representation of the arrangement of valence shell electrons in the molecule, which helps us understand the atoms' bond formations. The electrons that participate in bond formation are called the bonding pair of electrons, while those that don't are known as nonbonding pairs of electrons.
Lewis Dot Structure for CH4 #2 Find the number of "octet" electrons for the molecule. C: 8 octet electrons x 1 atom = 8 octet electrons H: 2 octet electrons x 4 .May 03, · A step-by-step explanation of how to draw the Lewis Structure Oxygen Gas (Dioxygen). For the O2 Lewis structure, calculate the total number of valence electrons for the ...
The Lewis Dot Structure for CH4 is shown above. These kinds of structures can also be shown by representing each of the bonds with two dots. Each atom in the bond has a full valence, with carbon having access to eight electrons and each hydrogen having access to two (this is why hydrogen only needs two).The covalent bonds between the C and the H are similar to the ones formed between two Hs ...
Dr. B. explains how to draw the Lewis dot structure for CH 4 (methane). The CH 4 Lewis Structure is one of the most frequently tested Lewis Structures.. Note that hydrogen atoms always go on the outside of a Lewis dot structure. This is because they can share a maximum of two electrons.
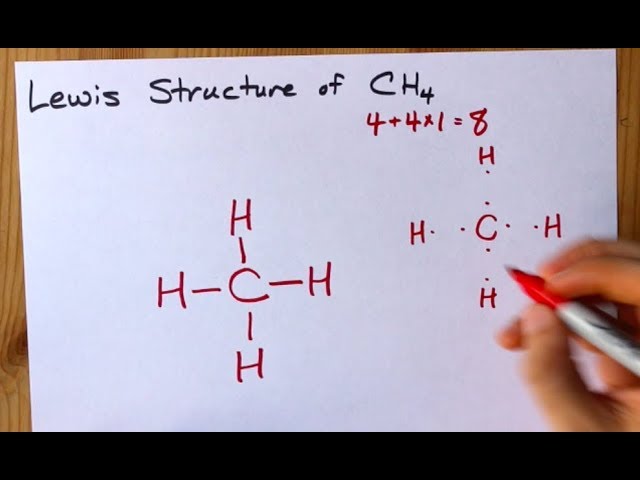
Lewis Structures for CH4. Step-by-step tutorial for drawing the Lewis Structure for CH4.Drawing the Lewis Structure for CH 4. For CH 4 you have a total of 8 total valence electrons.. Drawing the Lewis structure for CH 4 (named methane) requires only single diagramweb.net's one of the easier Lewis structures to draw.
Drawing the Lewis Structure for CH 4. For CH 4 you have a total of 8 total valence electrons.. Drawing the Lewis structure for CH 4 (named methane) requires only single bonds.It's one of the easier Lewis structures to draw. Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence electrons for a full outer shell.
Check me out: http://www.chemistnate.com
CH4 Lewis Structure, Molecular Geometry, and Hybridization. Methane or CH4 is a naturally occurring gas and relatively abundant on the Earth, making it an economically efficient fuel. As it releases more light and heat on burning, it is preferred more than coal, fossil fuel, or gasoline for energy production. It is one reason why overproduction ...
Ch4 Electron Dot Diagram. I will explain this with pictures, and some captions. This is just the five atoms in CH4, or Methane. I have drawn them above. The red one in the. Lewis Dot Structure for CH4 #2 Find the number of "octet" electrons for the molecule. C: 8 octet electrons x 1 atom = 8 octet electrons H: 2 octet electrons x 4 .
Nov 21, 2021 · MO Diagram of CCl4. A MO diagram is nothing but a representation of bonds that are formed within the atoms to form a compound. This diagram is based on Molecular orbital theory. With the help of a MO diagram, the existence of certain compounds can be explained. Here is the pictorial representation of how CCl4’s and CH4 MO diagram looks like.
CH4 Lewis Structure & Molecular Geometry. Methane ( CH4) is a colourless, odourless, and highly combustible gas that is utilized to generate energy and heat houses all over the world. CH4 Lewis structure comprises two different atoms: Carbon and hydrogen. It is a nonpolar molecule with a bond angle of 109.5° degrees.
Lewis Structure For Ch4, CH4 Lewis Structure How to Draw the Dot Structure for, Lewis Structure (+VSEPR) for CO2 YouTube, VSEPR PF5 Phosphorus Pentafluoride, [GM 1467] Dot Diagram Of Co2 Wiring Diagram
Ch4.5 Lewis Symbols and Lewis Structures. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 15 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 15.
How to Draw the Lewis Dot Structure for CH4: MethaneA step-by-step explanation of how to draw the CH4 Lewis Dot Structure (Methane).For the CH4 structure use...
In addition. Lewis Structures for CH4. Step-by-step tutorial for drawing the Lewis Structure for CH4. Lewis Dot Structure for CH4 #2 Find the number of "octet" electrons for the molecule. C: 8 octet electrons x 1 atom = 8 octet electrons H: 2 octet electrons x 4 . We show two ways to draw the CH4 Lewis structure, methane.
Answer: I would be lazy and look it up on the internet. But seriously, you have an electron pair between the C and each of the H's in the Lewis diagram a ala Why is that the correct diagram, you ask? First, each Hydrogen has only one electron to donate or share, and remember that Hydrogen's fil...




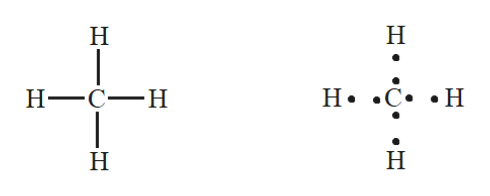
















0 Response to "41 lewis diagram for ch4"
Post a Comment