42 f2 2+ molecular orbital diagram
June 12, 2020 - Click here👆to get an answer to your question ✍️ 37. Draw molecular orbital diagram for F2 molecule. Also, give its electronic configuration, bond order and magnetic property. 138. Solve the following: Theoretical chemistry research group focusing on development of methods, and calculations in the areas of ionic liquids, photochemistry and catalysis
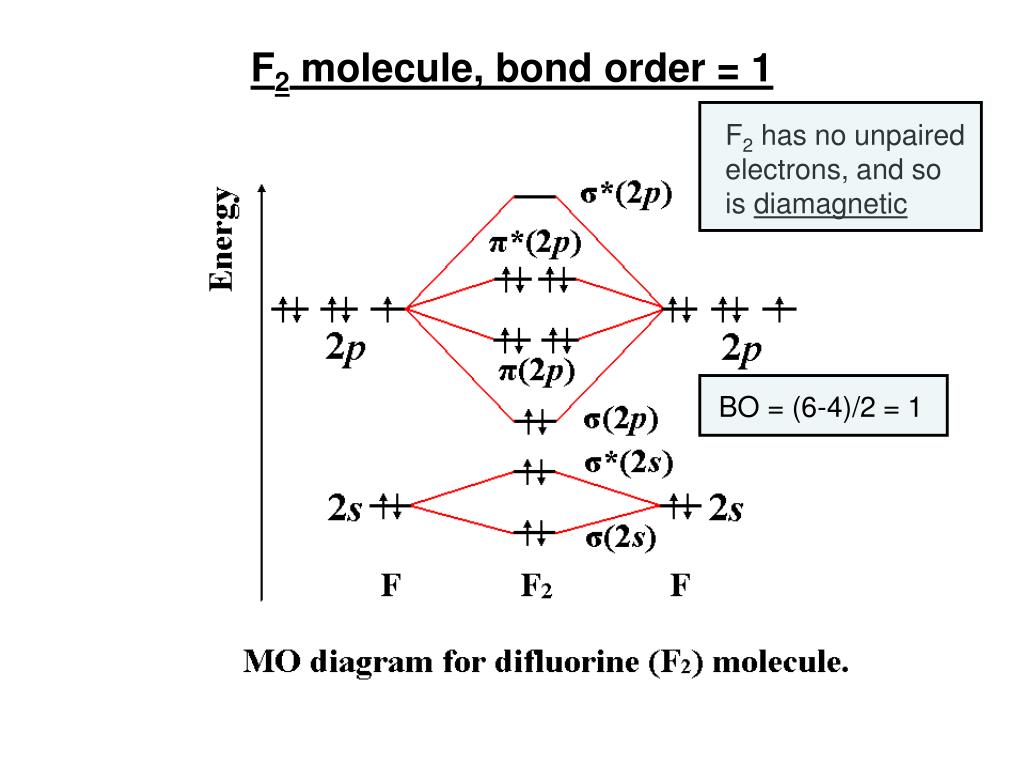
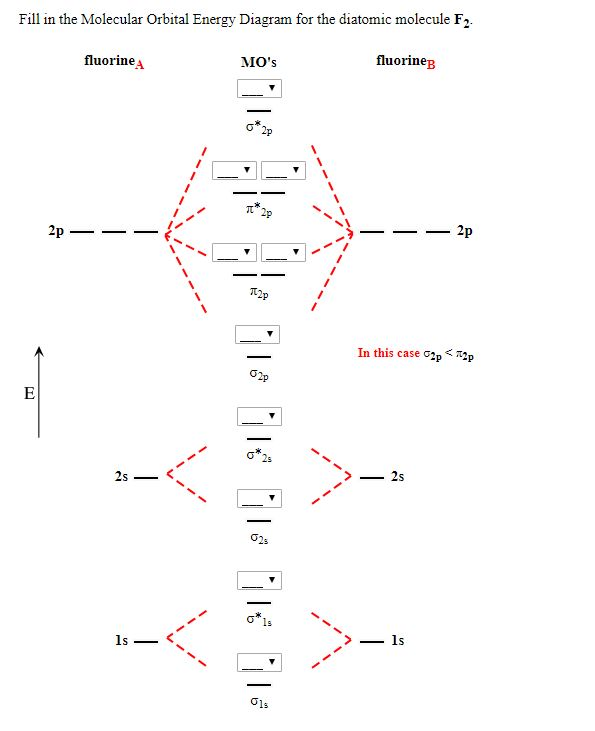
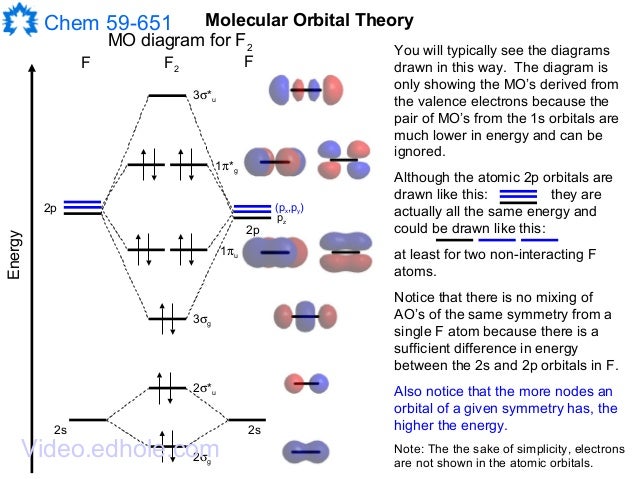
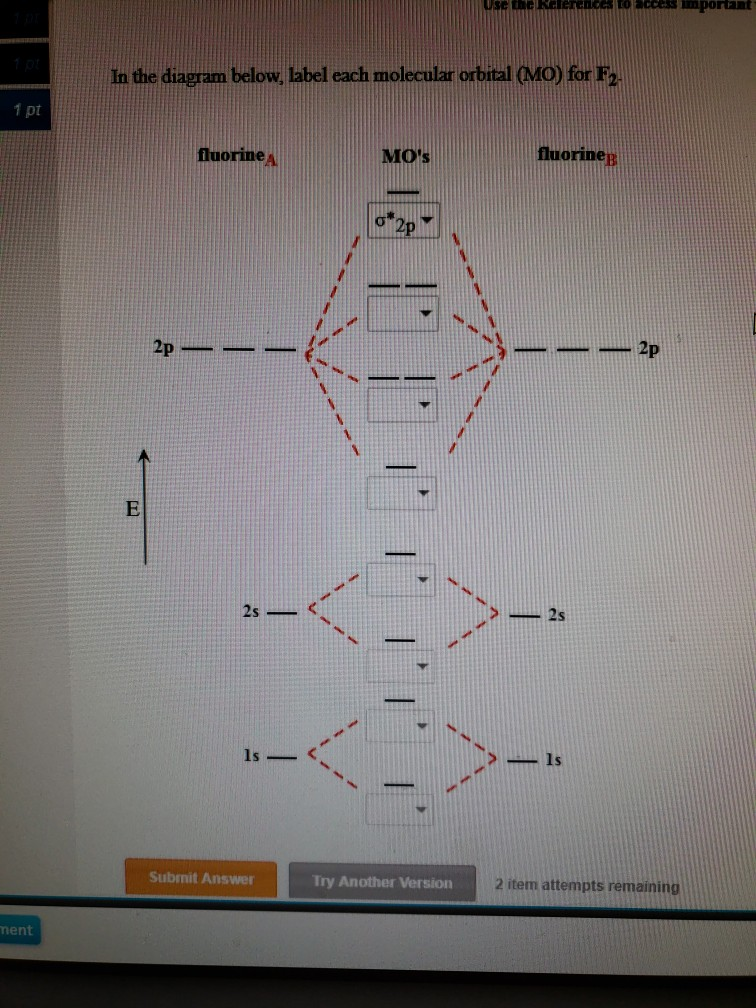
July 14, 2020 - N2 has the shortest bond because the bond order is 3 while O2 is 2 and F2 is 1. ... Draw the molecular orbital diagram for F2 with the atomic orbitals labeled and find the bond order.
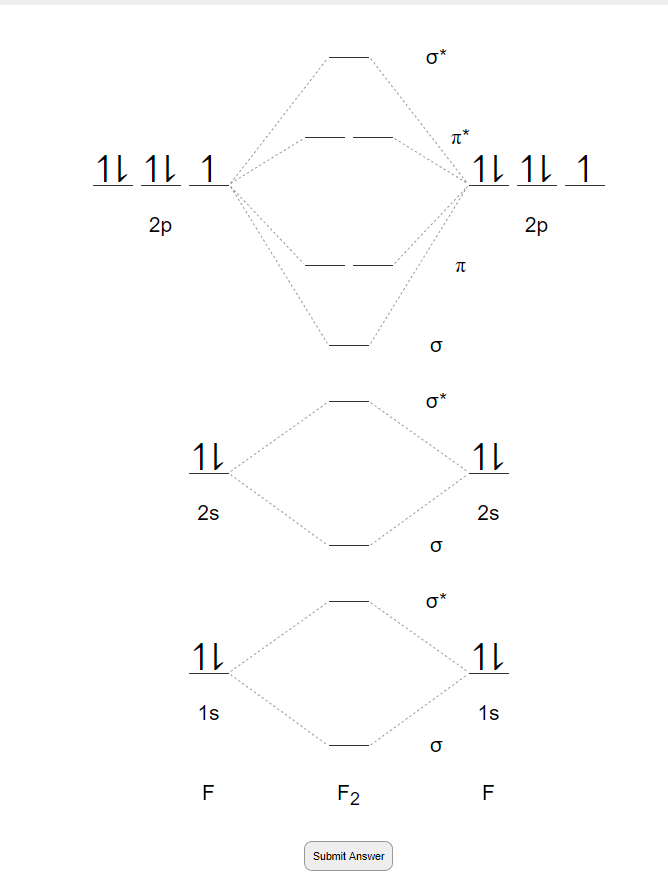
F2 2+ molecular orbital diagram
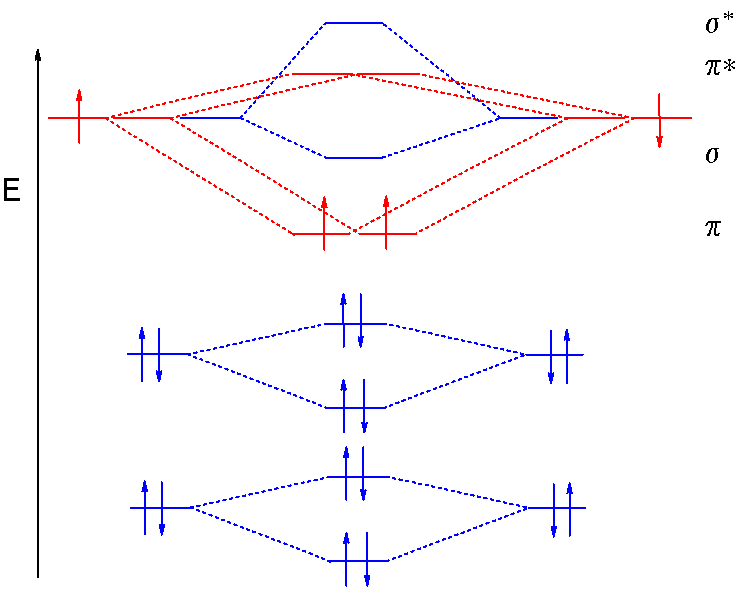
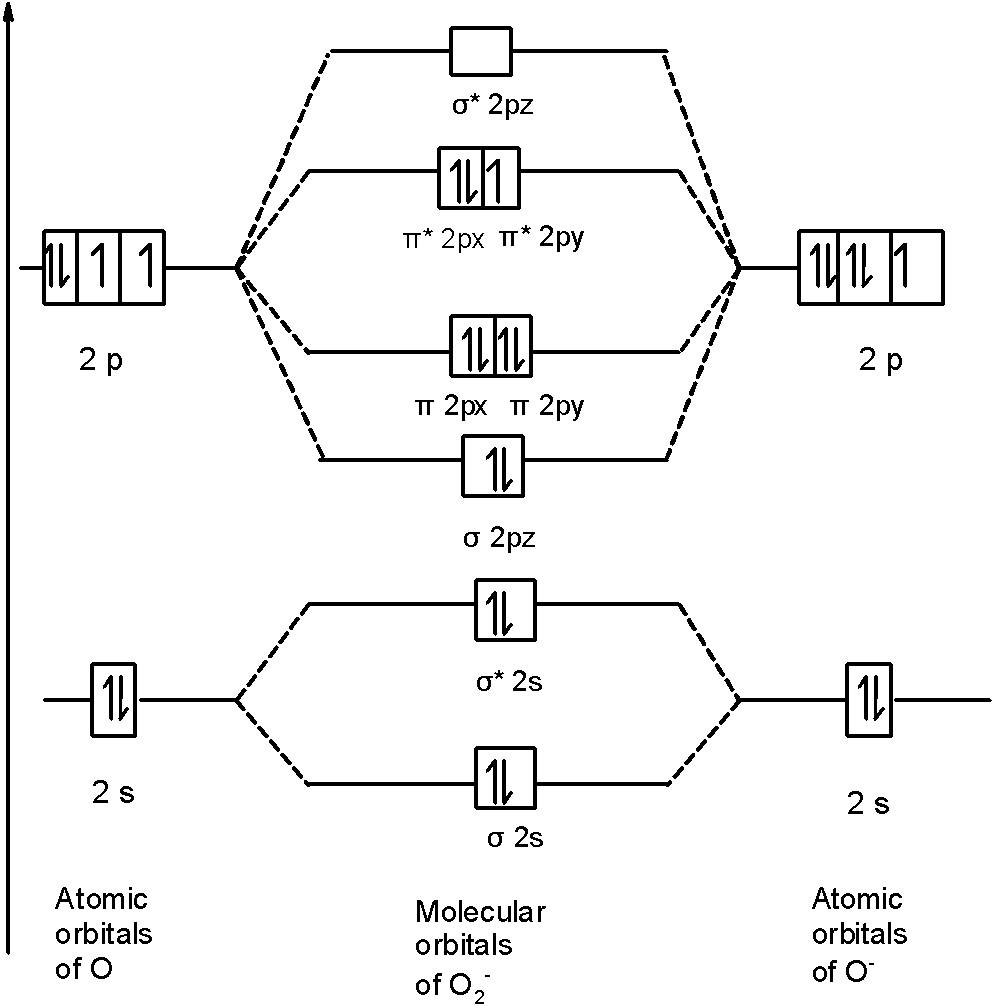
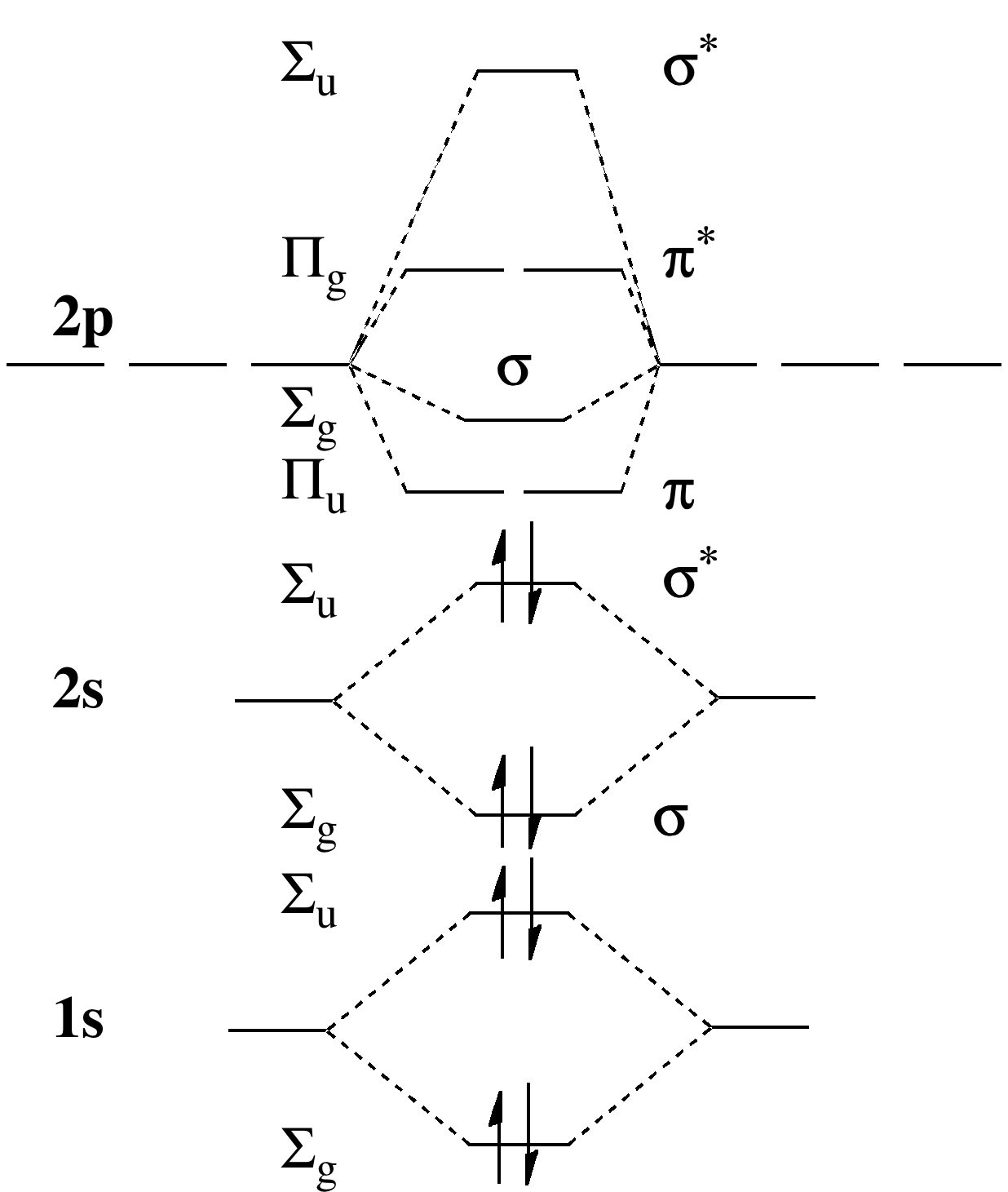
In order to continue enjoying our site, we ask that you confirm your identity as a human. Thank you very much for your cooperation February 3, 2018 - Answer (1 of 6): Here is the solution, > * For O2 molecule, > * For F2 molecule, Thanks for reading. August 15, 2020 - In O2 and F2, there is a crossover of the sigma and the pi ortbials: the relative energies of the sigma orbitals drop below that of the pi orbitals'. Information from the MO diagram justify O2's stability and show that it's bonding order is 2. The LUMO (lowest unoccupied molecular orbital) ...
F2 2+ molecular orbital diagram. What is the bond order of F22- according to molecular orbital theory? C Electron Configuration - 9 images - vsepr theory triiodide anion i3 expanded valence, 2 4 jjw 1 youtube, Answer to Draw a molecular orbital diagram for F2^2- . Calculate the bond order and magnetic behavior.... With nitrogen, we see the two molecular orbitals mixing and the energy repulsion. This is the reasoning for the rearrangement from a more familiar diagram. Notice how the σ from the 2p behaves more non-bonding like due to mixing, same with the 2s σ. This also causes a large jump in energy ...
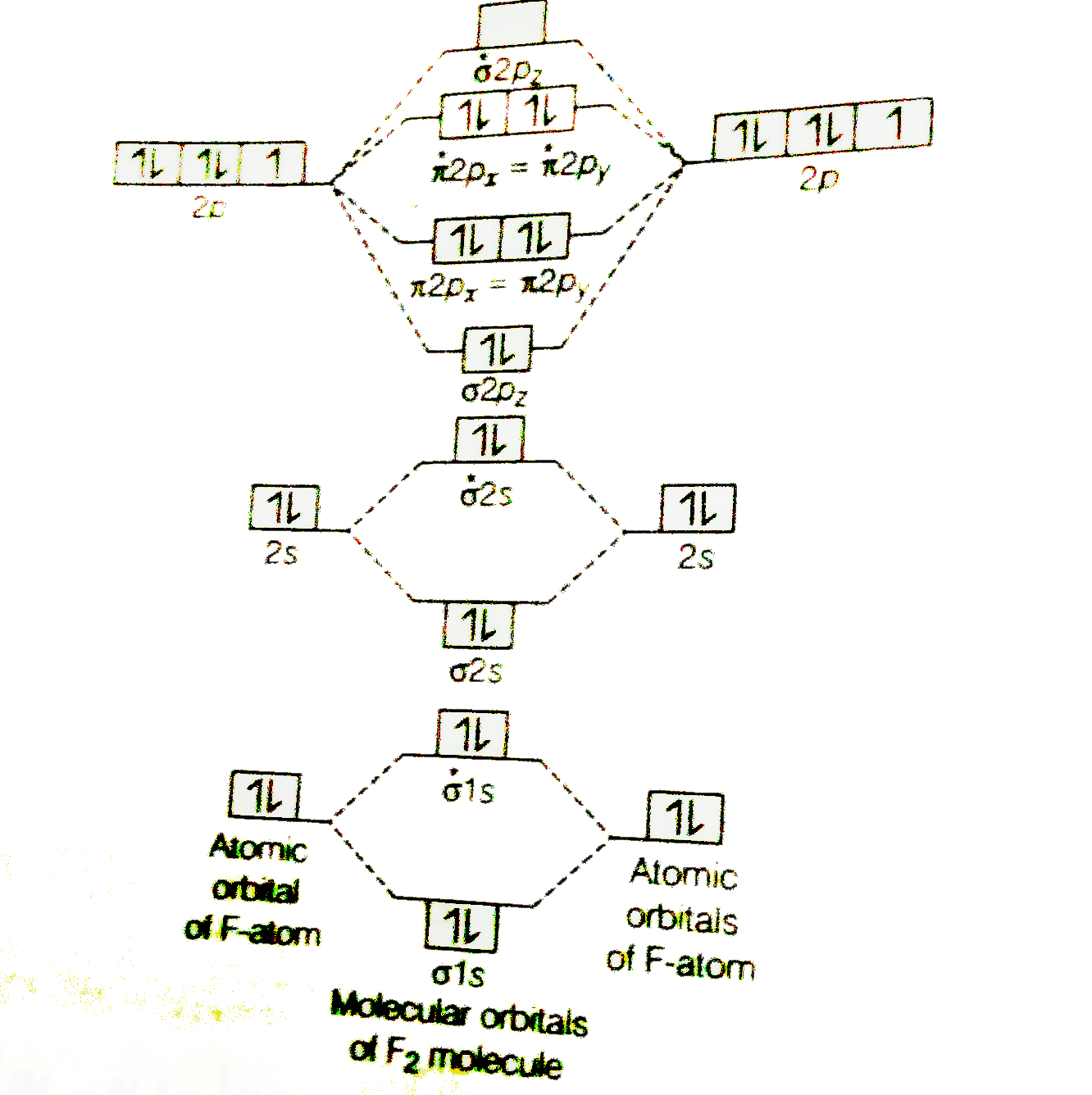
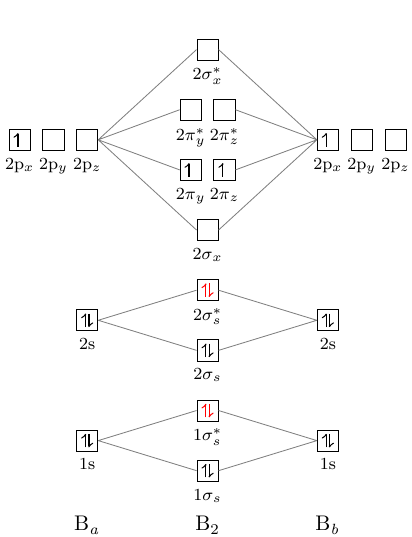
A planet is a large astronomical body that is not a star or stellar remnant.There are competing scientific definitions of a 'planet'. In the dynamicist definition adopted by the International Astronomical Union (IAU), a planet is a non-stellar body that is massive enough to be rounded by its own gravity, that directly orbits a star, and that has cleared its orbital zone of competing objects. Molecule me X Ground state electron ... Number F2 (01) 2 (01) 2 (021) (021) 2 (02) (Tap) 2 (720°) 2 paramagnetic diamagnetic Number Fz (Os) 2 (03°) 2 (02) 2 (023°) 2 (02) 2 (T2) 2 (1720°) 1 paramagnetic diamagnetic 1 2 3 4 5 Drag a number into each of the blank boxes above. Incorrect. Provide the molecular orbital diagram, predict the ... April 24, 2018 - Q. Use the molecular orbital diagram shown to determine which of the following is most stable.a. F22+b.Ne22+c. F22-d. O22+e. F2 January 21, 2016 - Molecular orbital diagrams chemistry x duration. This video is about mo diagram 2 f2. What Is The Molecular Orbital Diagram...
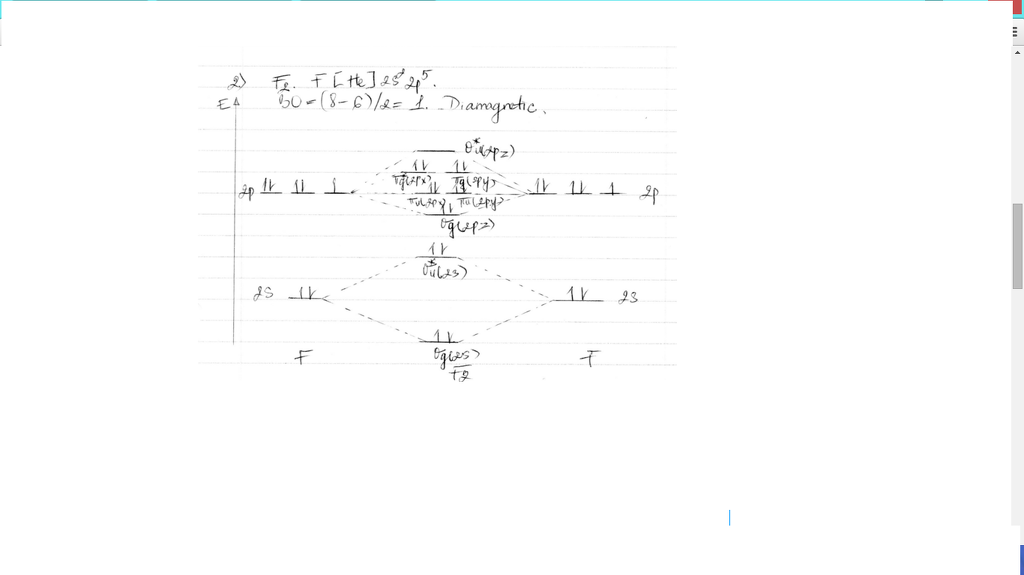
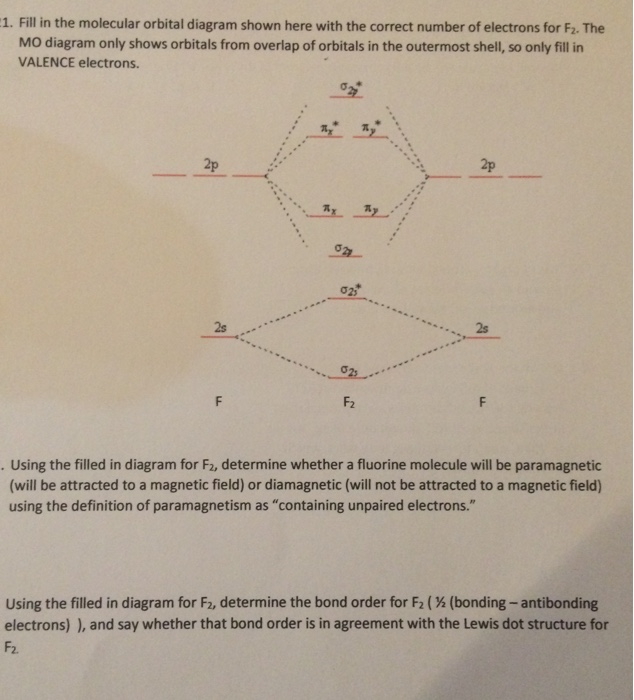
Draw molecular orbital diagram for F2 molecule Also gives its electronic configuration bond order and magnetic property November 23, 2015 - 12/20: Andy's paper on supported nanocrystal catalysts is published in Chem. Mater.! 10/20: Our joint paper with the Klimov group using ALD to produce CMOS circuit elements from CIS quantum dot films is published in Nature Comms. 9/20: Our paper with Adam Moule's group on electron tomography ... Problem: Use the molecular orbital diagram shown to determine which of the following is most stable.a. F22+b. Ne22+c. F22-d. O22+e. F2 Question: 22) Determine The Stability Of F22+ By Drawing A Molecular Orbital Energy Diagram That Includes The Valence Electrons (assume Ground State). Calculate The Bond Order Based On Your Diagram And Predict Whether F22 Should Be Diamagnetic Or Paramagnetic.
In order to continue enjoying our site, we ask that you confirm your identity as a human. Thank you very much for your cooperation
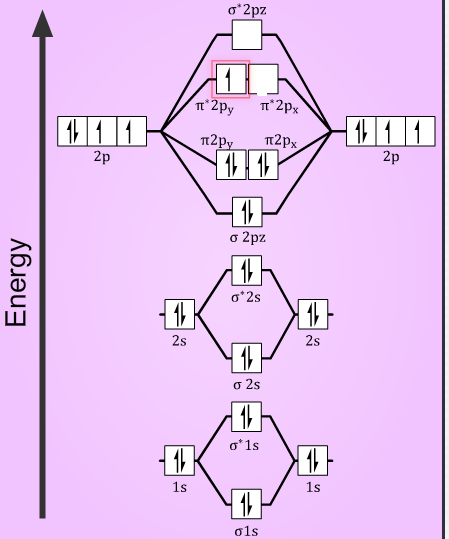
At the moment I'm learning about molecular orbital diagrams for homonuclear molecules, namely: B2, C2, N2, O2, F2, and Ne2. I understand that the energy of the 2p sigma bond is at a higher level for B2, C2, and N2, leading to the 2p sigma bond and the 2p pi bond switching places in the MO diagram (with 2p pi bond appearing under 2p sigma bond) for B, C, and N but not for O, F, or Ne. My lectures state that this is due to s and p mixing and my textbook states that it is due to electron repulsion ...
Our videos prepare you to succeed in your college classes. Let us help you simplify your studying. If you are having trouble with Chemistry, Organic, Physics, Calculus, or Statistics, we got your back! Our videos will help you understand concepts, solve your homework, and do great on your exams.
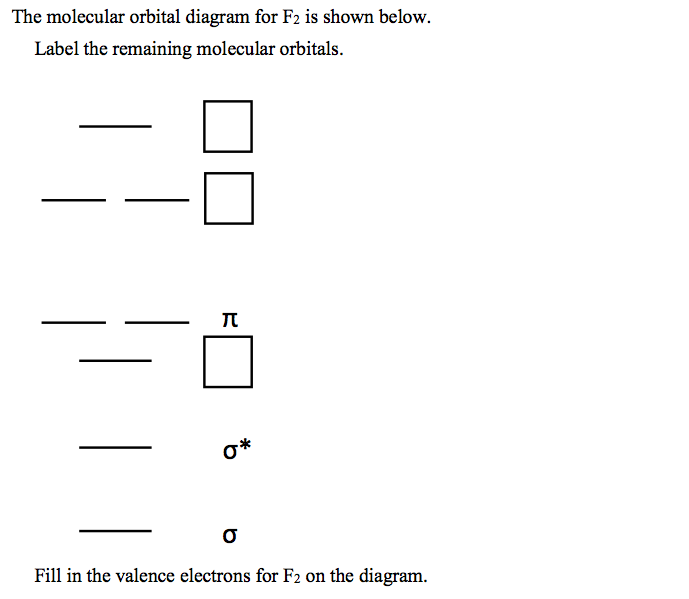
June 12, 2018 - You must generate a charge of +2. ... Below is a molecular orbital diagram for a fluorine molecule.
April 5, 2018 - Answer (1 of 5): The atomic number of fluorine is 9, so a (neutral) F2 molecule has a total of 18 electron, or 14 valence electrons (excluding the four 1s electrons). The (F2)- ion has one more valence electron, or 15. The orbital diagram for a diatomic molecule is To find the bond order, add th...
August 15, 2020 - In O2 and F2, there is a crossover of the sigma and the pi ortbials: the relative energies of the sigma orbitals drop below that of the pi orbitals'. Information from the MO diagram justify O2's stability and show that it's bonding order is 2. The LUMO (lowest unoccupied molecular orbital) ...
February 3, 2018 - Answer (1 of 6): Here is the solution, > * For O2 molecule, > * For F2 molecule, Thanks for reading.
In order to continue enjoying our site, we ask that you confirm your identity as a human. Thank you very much for your cooperation


















0 Response to "42 f2 2+ molecular orbital diagram"
Post a Comment