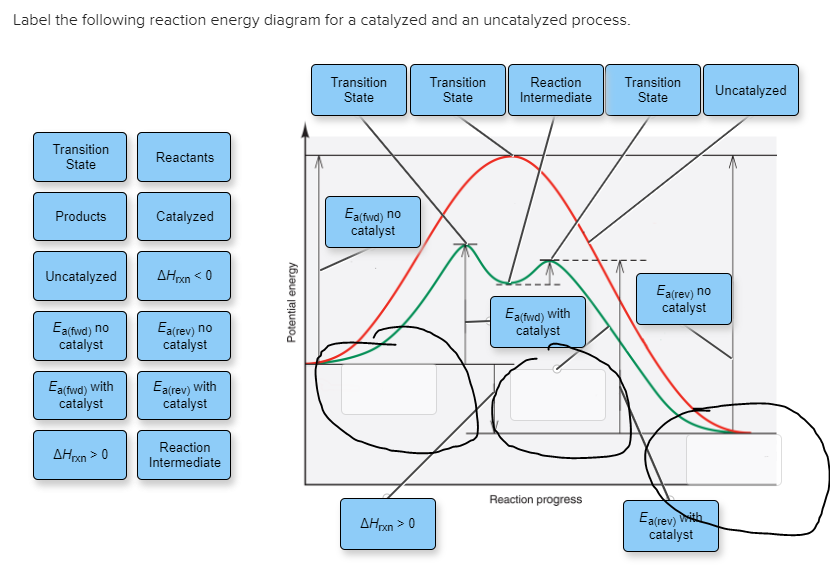
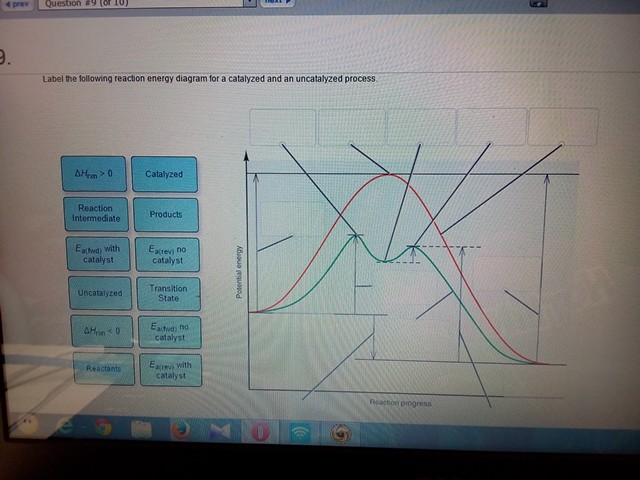
38 Label The Following Reaction Energy Diagram For A Catalyzed And An Uncatalyzed Process.
Solved Label the following reaction energy diagram for a Chemistry questions and answers. Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. A > 0 Eatwa) no catalyst Exter, with catalyst Reactants Esinev) no Eastw with catalyst Potential energy Mono Products Uncatalyzed Catalyzed Transition State Reaction Intermediate Reaction progress Reset Zoom. 33 Label The Following Reaction Energy Diagram For A ... Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. Label the energy diagram and answer the question that follows. The solid line in the energy diagram represents changes in energy as the reactant is converted to product under standard conditions. Which equation below indicates how the presence of an enzyme affects the activation energy of the reaction catalyzed versus uncatalyzed. The mechanism may be different for a catalyzed reaction reaction vs the ...
Solved Label the following reaction energy diagram for a Transcribed image text: Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. AHx>0 AH 0 Eartwd) with catalyst Reaction ...
Label the following reaction energy diagram for a catalyzed and an uncatalyzed process.
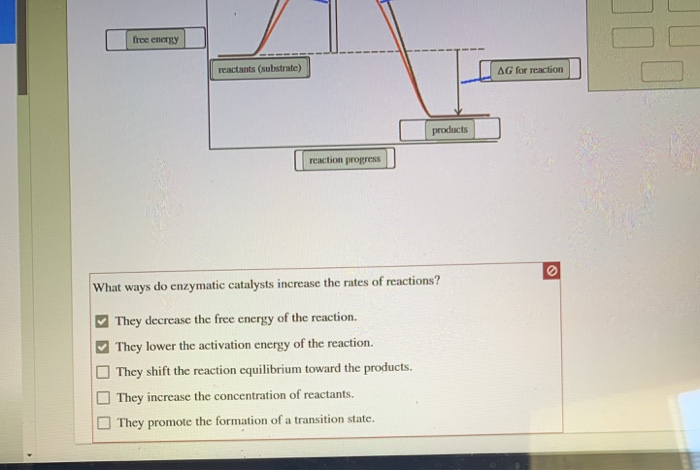
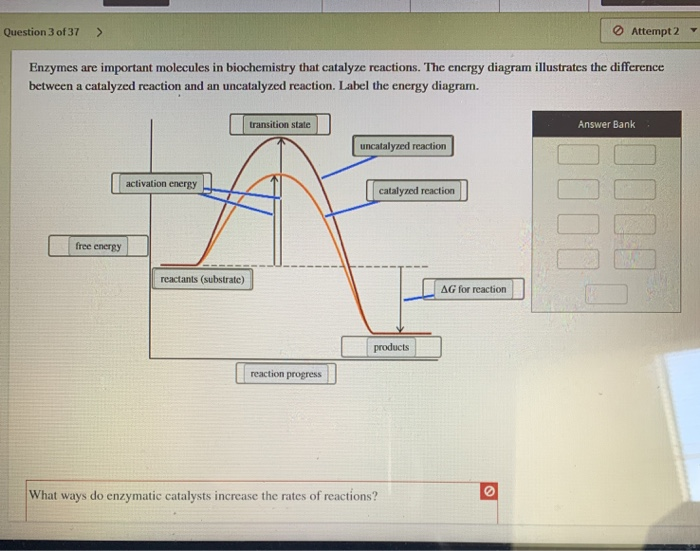
Energy Diagram Catalyzed Vs Uncatalyzed Reaction Below is an energy diagram illustrating the difference in a catalyzed reaction versus an uncatalyzed reaction. Label the energy diagram and answer the question that follows%(1). Catalyzed reactions have a lower activation energy (rate-limiting free energy of activation) than the corresponding uncatalyzed reaction, resulting in a higher reaction rate at the same temperature and for the same reactant concentrations. Biochemistry Homework 5 questions Flashcards | Quizlet Below is an energy diagram illustrating the difference between a catalyzed reaction and an uncatalyzed reaction. Label the energy diagram and answer the question that follows. What ways do enzymatic catalysts increase the rates of reactions? Solved Label the following reaction energy diagram for a Transcribed image text: Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. Uncatalyzed Catalyzed Eare) with catalyst ...
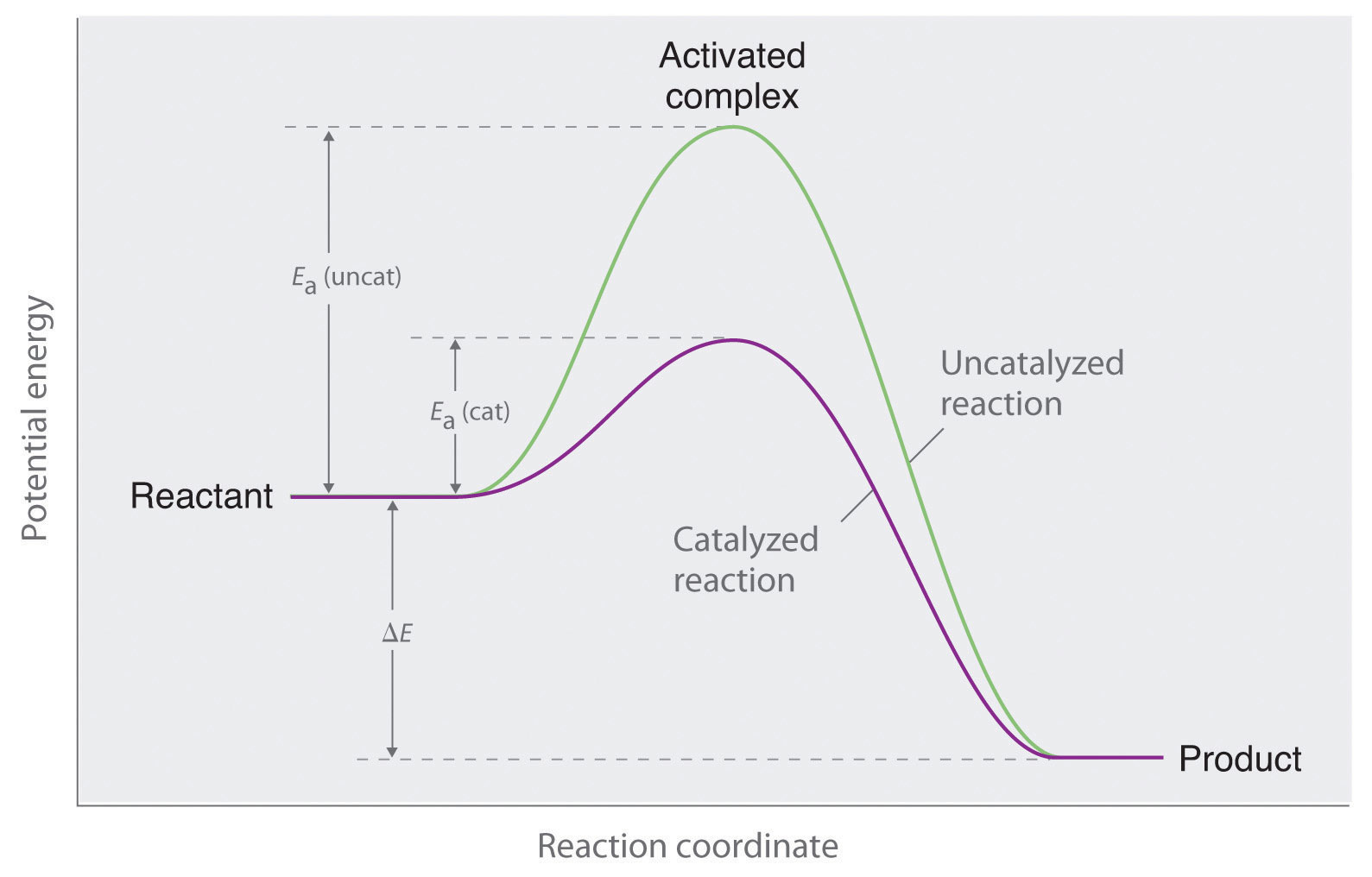
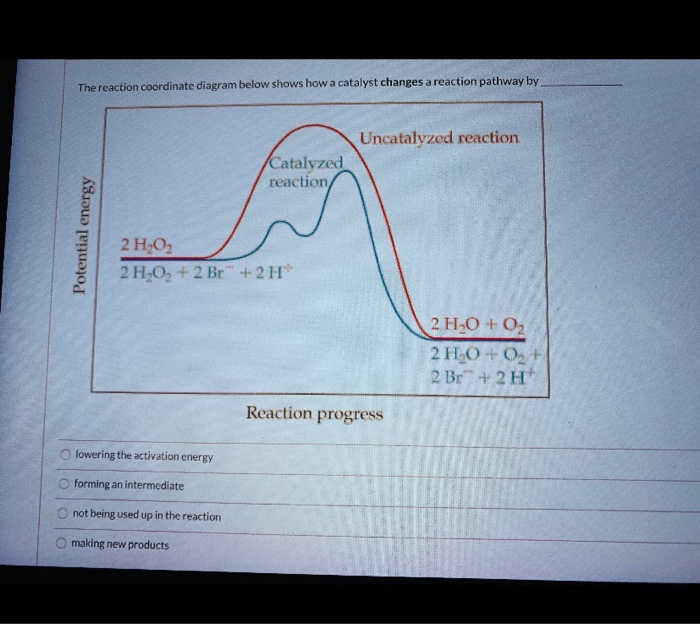
Label the following reaction energy diagram for a catalyzed and an uncatalyzed process.. Solved Label the following reaction energy diagram for a Show transcribed image text. Best Answer. This is the best answer based on feedback and ratings. 89% (38 ratings) Transcribed image text: Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. Previous question Next question. Solved Label the following reaction energy diagram for a Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. AHpXn > 0 Ealfwd) no catalyst Earrev) with catalyst Reactants Earrev) no catalyst Eaſfwd) with catalyst Potential energy AHpxn < 0 Products Uncatalyzed Catalyzed Transition State Reaction Intermediate Reaction progress Reset Zoom 12.7 Catalysis - Chemistry - opentextbc.ca One such reaction is catalytic hydrogenation, the process by which hydrogen is added across an alkene C=C bond to afford the saturated alkane product. A comparison of the reaction coordinate diagrams (also known as energy diagrams) for catalyzed and uncatalyzed alkene hydrogenation is shown in Figure 1. Figure 1. PDF AP CHEMISTRY 2006 SCORING GUIDELINES - College Board (d) Consider the four reaction-energy profile diagrams shown below. (i) Identify the two diagrams that could represent a catalyzed and an uncatalyzed reaction pathway for the same reaction. Indicate which of the two diagrams represents the catalyzed reaction pathway for the reaction. Diagram 1 represents a catalyzed pathway and diagram 2
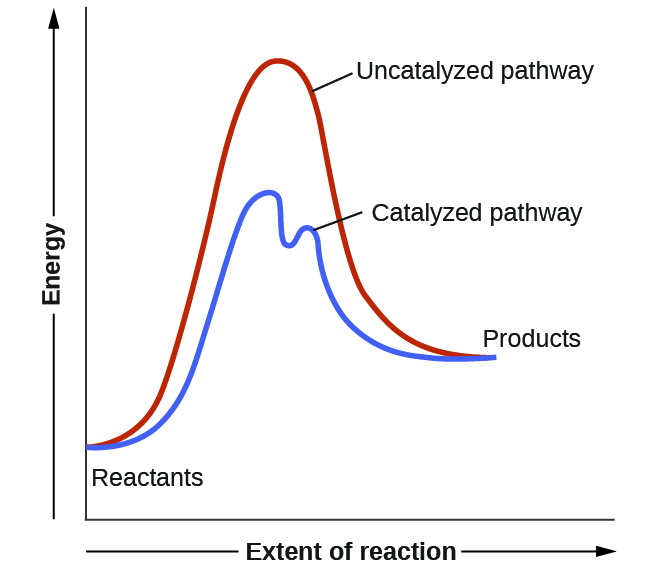
Organic chem please help? Label each reactant and product in this reaction as a brønsted acid or base. o ultimo exorcismo parte 3 ? Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. meaning of abnosis? Diagram The Reaction Course Of An Exergonic Reaction Free ... Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. Catalyzed reactions have a lower activation energy rate limiting free energy of activation than the corresponding uncatalyzed reaction resulting in a higher reaction rate at the same temperature and for the same reactant concentrations. PDF ap07 chemistry q6 - College Board (e) Using the axes provided below, draw the complete potential-energy diagram for both the catalyzed and uncatalyzed reactions. Clearly label the curve that represents the catalyzed reaction. One point is earned for an uncatalyzed reaction curve that must show that E a > 0 and ΔH < 0. One point is earned for a catalyzed reaction curve that must show E Answered: В (ОН)2 Pd(OAc)2, PPH3 aq Na2CO3 Br R'… | bartleby Draw and label a potential energy coordinate diagram that shows the difference between the Suzuki reaction you studied in an uncatalyzed versus catalyzed process. Assume that the Ea for the uncatalyzed reaction is 32 kcal/mole and the DGo is -7 kcal/mole.
6.7: Energy Diagrams - Chemistry LibreTexts 6.7: Energy Diagrams. You may recall from general chemistry that it is often convenient to describe chemical reactions with energy diagrams. In an energy diagram, the vertical axis represents the overall energy of the reactants, while the horizontal axis is the ' reaction coordinate ', tracing from left to right the progress of the reaction ... Solved Label the following reaction energy diagram for a Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. Reactants State Products Catalyzed Uncatalyzed | | ΔHnn < 0 Ea (lwd) no catalyst Ea (rev) no catalyst Fattwo) with Ea (rev) With catalyst catalyst Reaction AHrxn 0 Intermediate Reaction progress. Question: Label the following reaction energy diagram for a ... HW Solutions #9 - Chemistry LibreTexts 1. Answer the following questions based on the potential energy diagram shown here: Does the graph represent an endothermic or exothermic reaction? Label the position of the reactants, products, and activated complex. Determine the heat of reaction, ΔH, (enthalpy change) for this reaction. Determine the activation energy, E a for this reaction. Answered: (a) Draw an activation energy diagram… | bartleby Science Biology Q&A Library (a) Draw an activation energy diagram for this reaction under catalyzed and uncatalyzed conditions and explain what it means for the activation energy to be lowered from 18 to 13 kcal/mol by ferric ions but from 18 to 7 kcal/mol by catalase. Please draw in the space provided below. (b) Suggest two properties of catalase that make it a more suitable intracellular ...
Energy Diagram Catalyzed Vs Uncatalyzed Reaction - Blogger Energy diagram catalyzed vs uncatalyzed reaction. There is no effect on the energy of the reactants or the products. Learn vocabulary terms and more with flashcards games and other study tools. Models of enzyme substrate interaction. Label the following reaction energy diagram for a catalyzed and an uncatalyzed process.
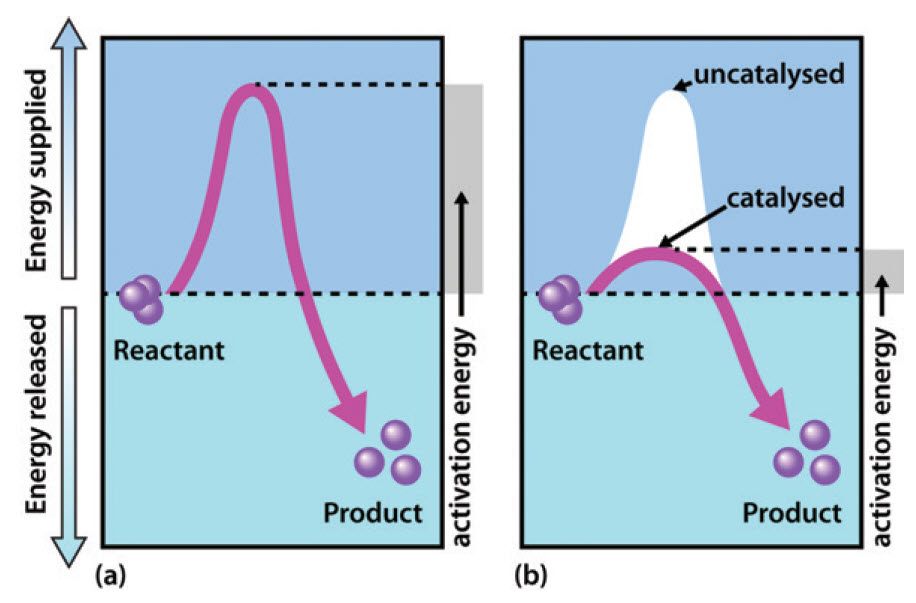
BioChemistry Ch 8 Flashcards | Quizlet The enzyme urease catalyzes the hydrolysis of urea to ammonia plus carbon dioxide. At 21 ∘C∘C the uncatalyzed reaction has an activation energy of about 125 kJ/mol, whereas in the presence of urease the activation energy is lowered to about 46 kJ/mol.
Energy Diagram Catalyzed Vs Uncatalyzed Reaction Below is an energy diagram illustrating the difference in a catalyzed reaction versus an uncatalyzed reaction. Label the energy diagram and answer the question that follows% (1). The decomposition of hydrogen peroxide is exothermic. The reaction is catalyzed by iodide ion. The equation for the uncatalyzed reaction is.
Chapter 8 BIOCHEM Flashcards - Quizlet The graph presents three activation energy profiles for a chemical reaction (the hydrolysis of sucrose): an uncatalyzed reaction, and the same reaction catalyzed by two different enzymes. most product formed: rxn cat by enzyme B. middle: reaction cat by enzyme Z. Least product formed: uncatalyzed rxn.
Label the following reaction energy diagra... | Clutch Prep Hi there! We’re asked to label the following reaction energy diagram for a catalyzed and an uncatalyzed process. Recall that a catalyst speeds up a reaction by lowering the activation energy needed. This means that: The red line corresponds to the uncatalyzed reaction (steeper hill) The green line corresponds to the catalyzed reaction (lower ...
PDF Graph Review Wed - ISD 622 1. Below is an energy diagram for a particular process. One curve represents the energy profile for the uncatalyzed reaction, and the other curve represents the energy profile for the catalyzed reaction. Course reaction a. N%ich curve has the geater activation energy? Curve 1 or cunre 2? b. WIIich curve has the greater energy chanoe, AE? b)curve 2
Solved Label the following reaction energy diagram for a Transcribed image text: Label the following reaction energy diagram for a catalyzed and an uncatalyzed process Transition State Reactants Products Catalyzed ...
40 label the energy diagram for a two-step reaction ... Label the energy diagram for a two-step reaction. Draw and label potential energy diagram for the reaction including a molecular structure that could represent an activated complex. The activated complex would show an unstable association of one CH 4(g) molecule and O 2(g) molecule with partial bonds. Check Your Solution The potential energy diagram should match the given information. Label ΔH as positive or negative.
Solved Label the following reaction energy diagram for a ... Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. Transition State Transition State Transition intermediate Reaction Intermediate Transition State Transition Uncatalyzed Transition State Reactants Products Catalyzed Ea (fwd) no catalyst Uncatalyzed AHxn < 0 Potential energy Ea (rev) no catalyst Ea (fwd) no catalyst Ea (rev)
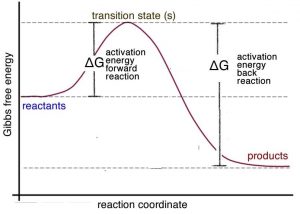
Biochemistry I Chapter 11 Problems Flashcards - Quizlet *Figure 1 shows a reaction with a negative free energy change and Figure 2 shows a reaction with a positive free energy change. Reactions with a negative free energy change are termed spontaneous. Which one of the following statements about enzyme catalyzed reactions is FALSE?
Solved Label the following reaction energy diagram for a Transcribed image text: Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. Uncatalyzed Catalyzed Eare) with catalyst ...
Biochemistry Homework 5 questions Flashcards | Quizlet Below is an energy diagram illustrating the difference between a catalyzed reaction and an uncatalyzed reaction. Label the energy diagram and answer the question that follows. What ways do enzymatic catalysts increase the rates of reactions?
Energy Diagram Catalyzed Vs Uncatalyzed Reaction Below is an energy diagram illustrating the difference in a catalyzed reaction versus an uncatalyzed reaction. Label the energy diagram and answer the question that follows%(1). Catalyzed reactions have a lower activation energy (rate-limiting free energy of activation) than the corresponding uncatalyzed reaction, resulting in a higher reaction rate at the same temperature and for the same reactant concentrations.








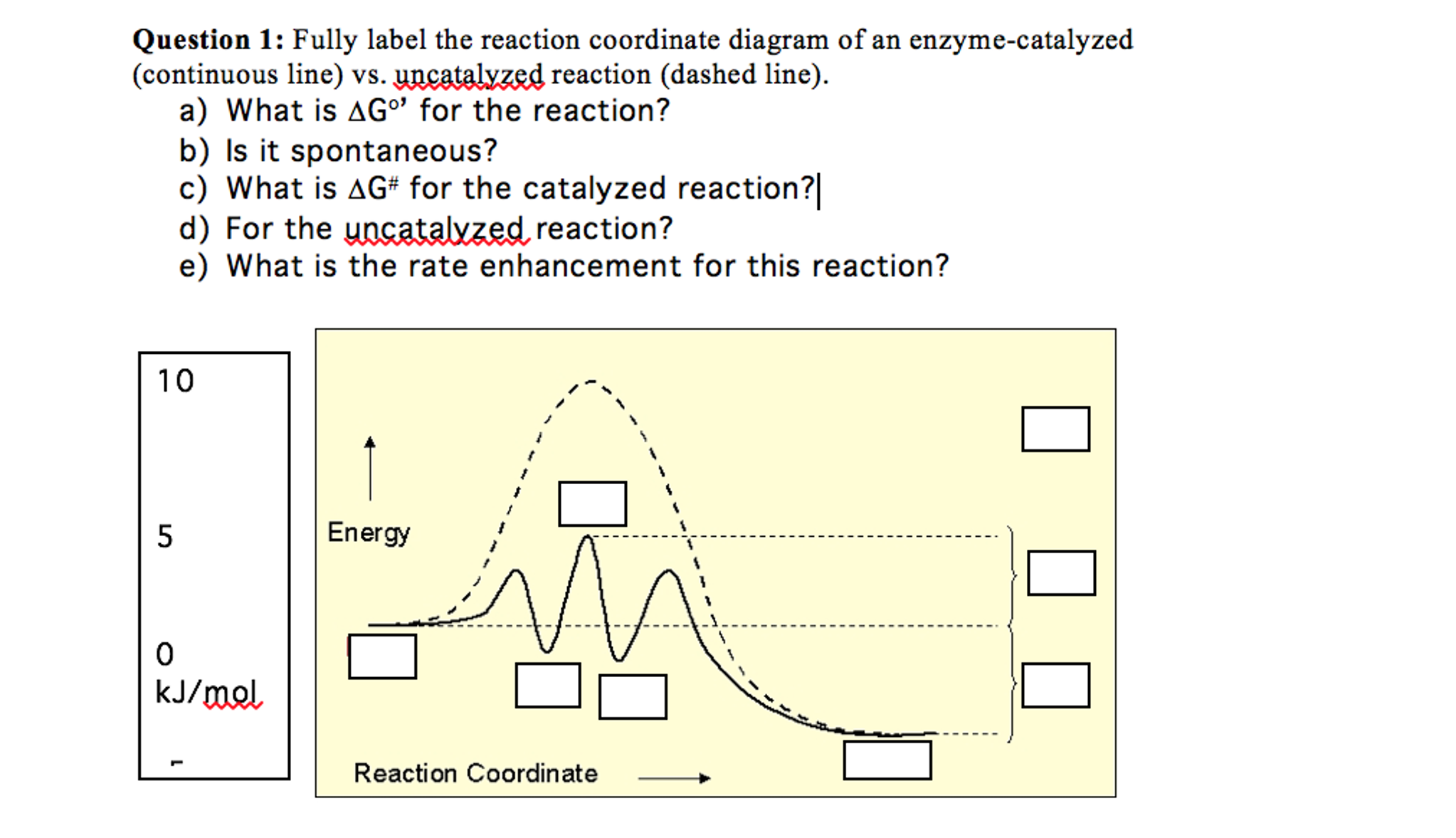
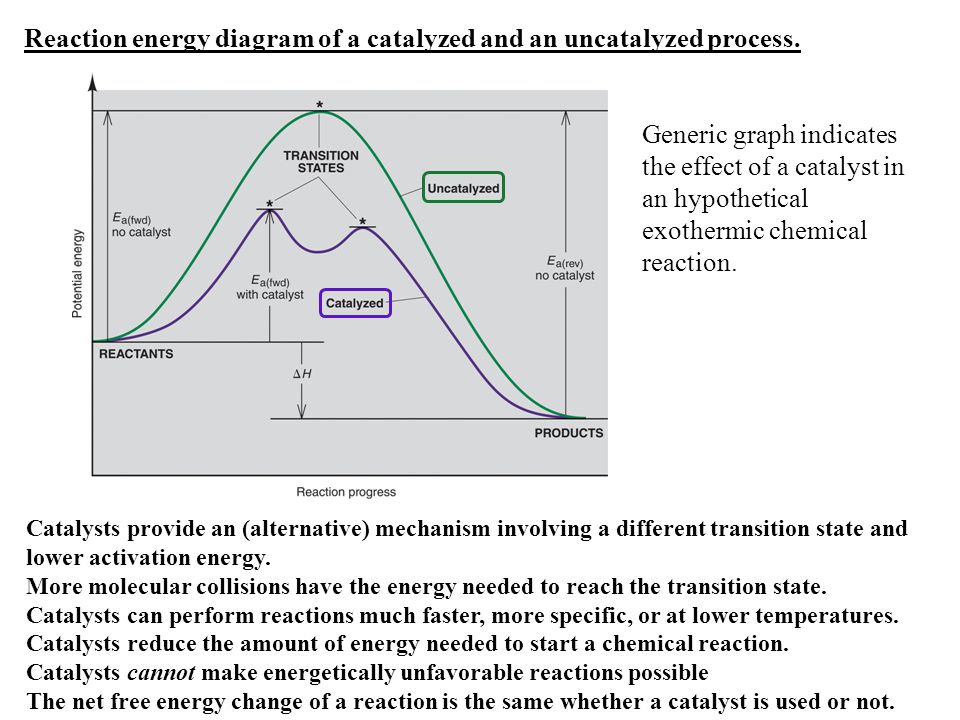
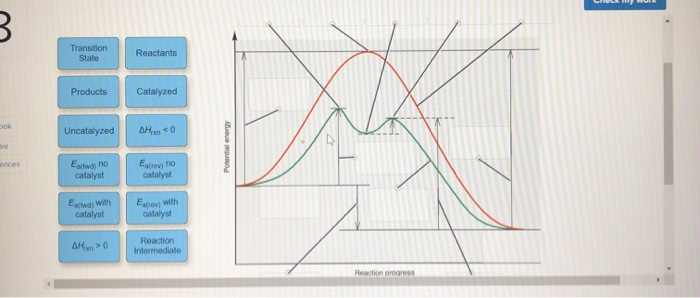
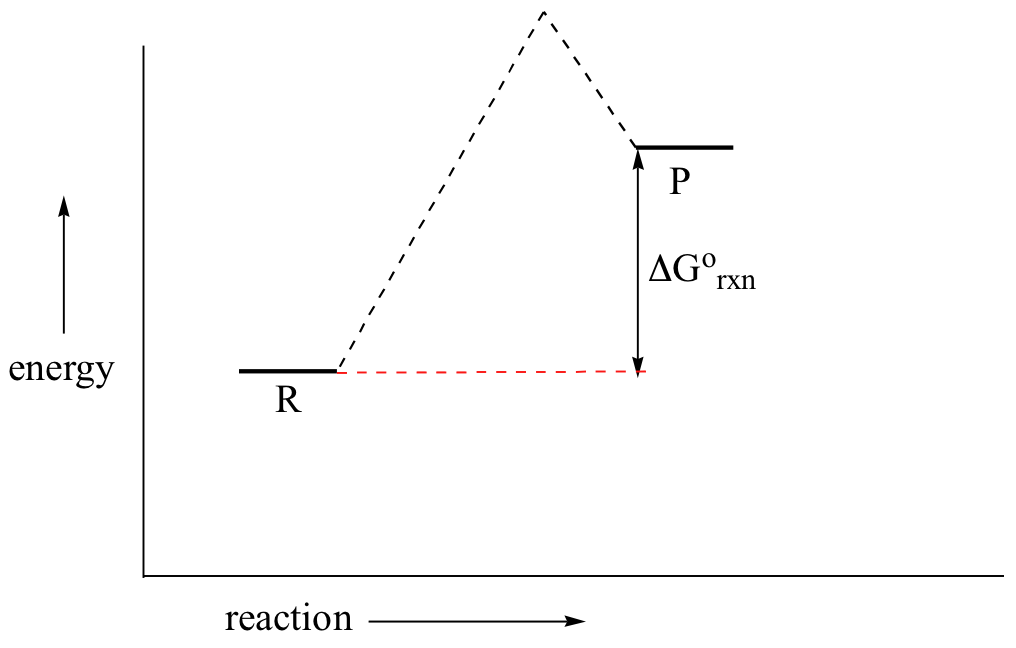







0 Response to "38 Label The Following Reaction Energy Diagram For A Catalyzed And An Uncatalyzed Process."
Post a Comment